Chemistry > GIZMOS > Gizmos_Student Exploration: Moles 2020 | Gizmos_Student Exploration: Moles_Graded A (All)
Gizmos_Student Exploration: Moles 2020 | Gizmos_Student Exploration: Moles_Graded A
Document Content and Description Below
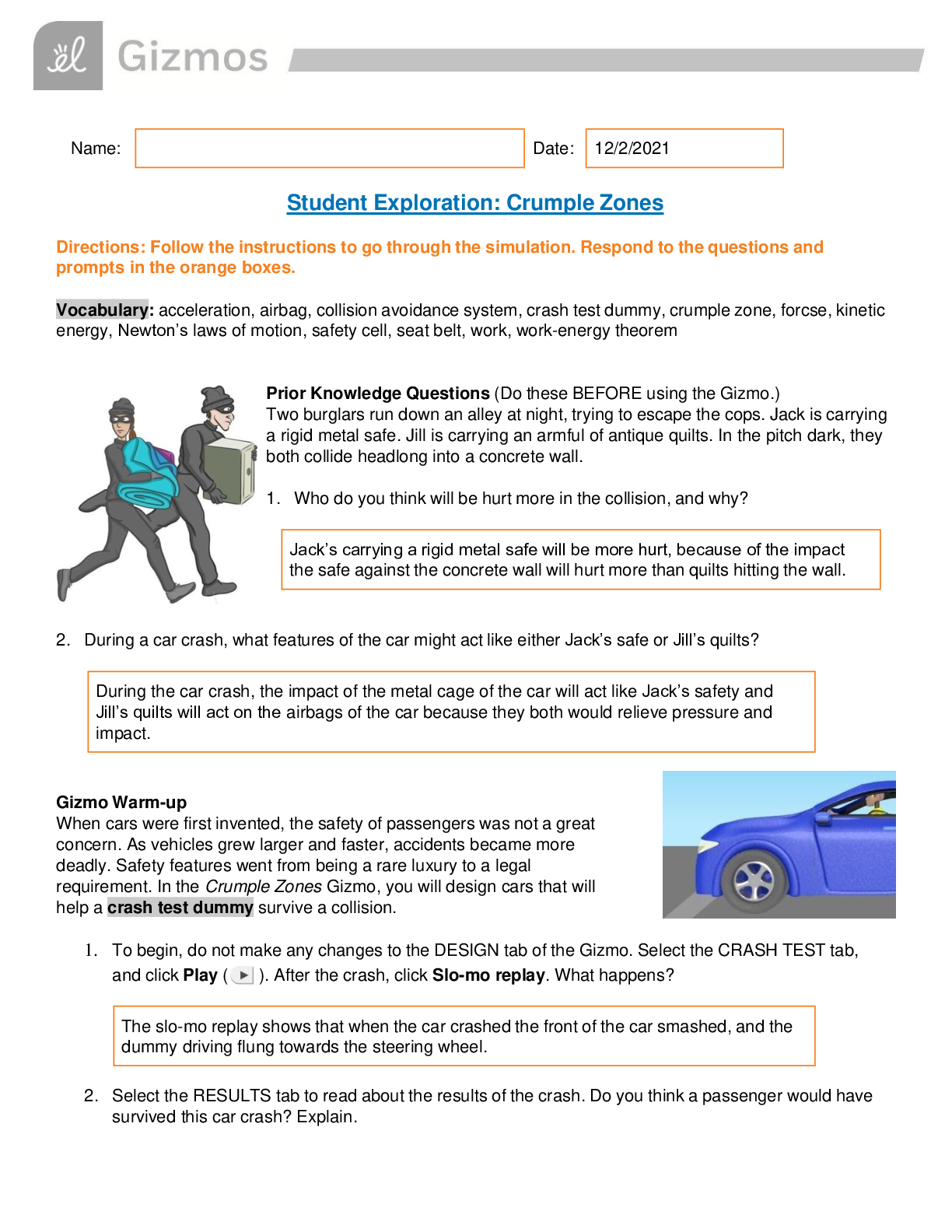
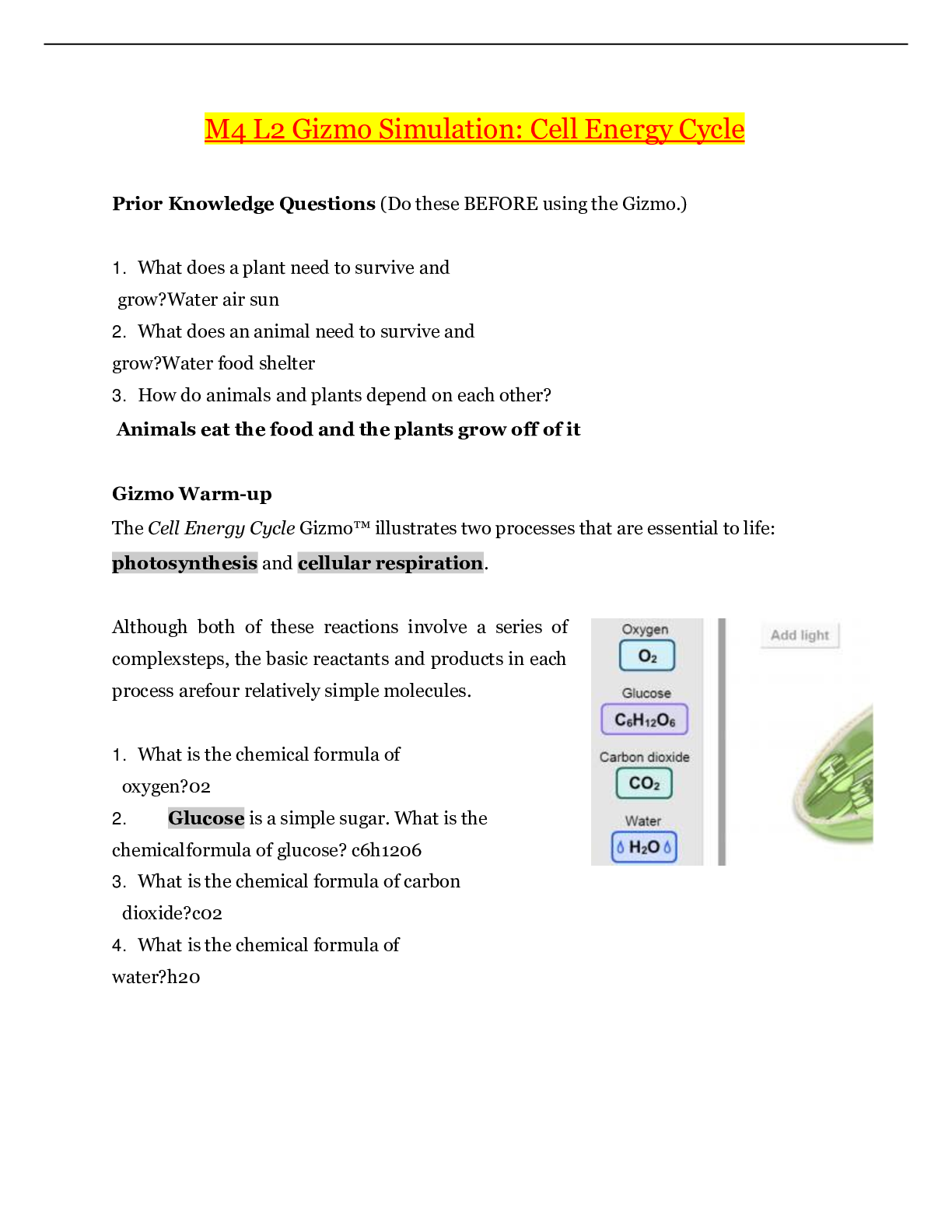
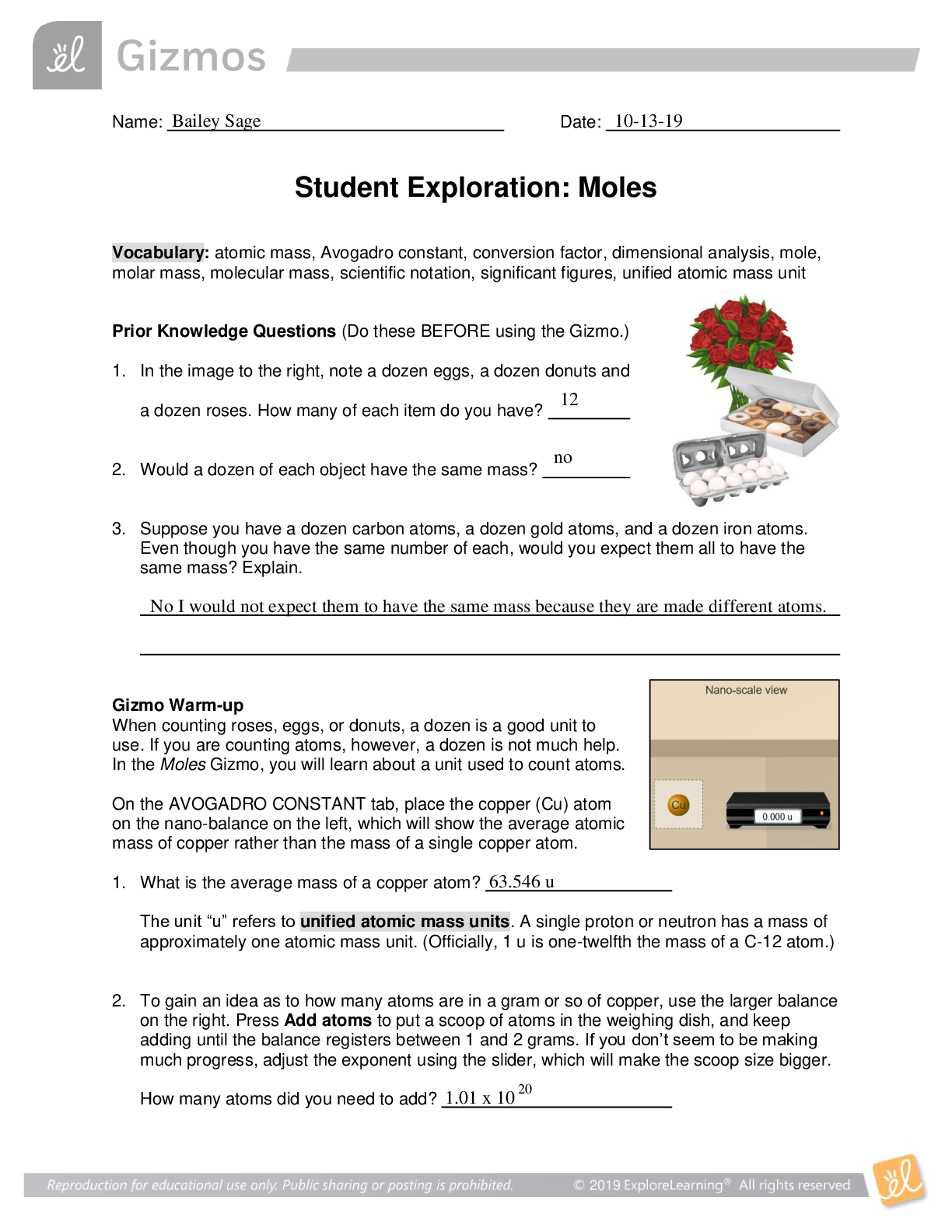
Name: Date: Student Exploration: Moles Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabulary: atomic m... ass, Avogadro constant, conversion factor, dimensional analysis, mole, molar mass, molecular mass, scientific notation, significant figures, unified atomic mass unit Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. In the image to the right, note a dozen eggs, a dozen donuts and a dozen roses. How many of each item do you have? 2. Would a dozen of each object have the same mass? 3. Suppose you have a dozen carbon atoms, a dozen gold atoms, and a dozen iron atoms. Even though you have the same number of each, would you expect them all to have the same mass? Explain. Gizmo Warm-up When counting roses, eggs, or donuts, a dozen is a good unit to use. If you are counting atoms, however, a dozen is not much help. In the Moles Gizmo, you will learn about a unit used to count atoms. On the AVOGADRO CONSTANT tab, place the copper (Cu) atom on the nano-balance on the left, which will show the average atomic mass of copper rather than the mass of a single copper atom. 1. What is the average mass of a copper atom? The unit “u” refers to unified atomic mass units. A single proton or neutron has a mass of approximately one atomic mass unit. (Officially, 1 u is one-twelfth the mass of a C-12 atom.) 2. To gain an idea as to how many atoms are in a gram or so of copper, use the larger balance on the right. Press Add atoms to put a scoop of atoms in the weighing dish, and keep adding until the balance registers between 1 and 2 grams. If you don’t seem to be making much progress, adjust the exponent using the slider, which will make the scoop size bigger. How many atoms did you need to add? Activity A: Molar Mass Get the Gizmo ready: ● Select the AVOGADRO CONSTANT tab. ● Turn on Show hints and check that Copper (Cu) is selected. Introduction: Since atoms are so tiny, chemists have devised a unit known as the mole. A mole represents a macroscopic quantity of matter that can be used in the laboratory. One mole of any element has the same mass in grams as its atomic mass in u. Question: How many particles are in a mole? 1. Explore: Note the average atomic mass of copper on the nano-balance. Add atoms to the larger balance until it registers the same number (in g) as the reading on the nano-balance (in u). Use the Exponent slider to help get the correct amount. Stop adding atoms when the readings on both balances match exactly (to the nearest 0.001 g). How many atoms did you need to add? [Show More]
Last updated: 2 years ago
Preview 1 out of 9 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 03, 2021
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Apr 03, 2021
Downloads
0
Views
122