Physics > GIZMOS > Gizmos _Feel the Heat Student Exploration_2021 | PHY 111 Gizmos_Feel the Heat Student Exploration_Qu (All)
Gizmos _Feel the Heat Student Exploration_2021 | PHY 111 Gizmos_Feel the Heat Student Exploration_Questions Only
Document Content and Description Below
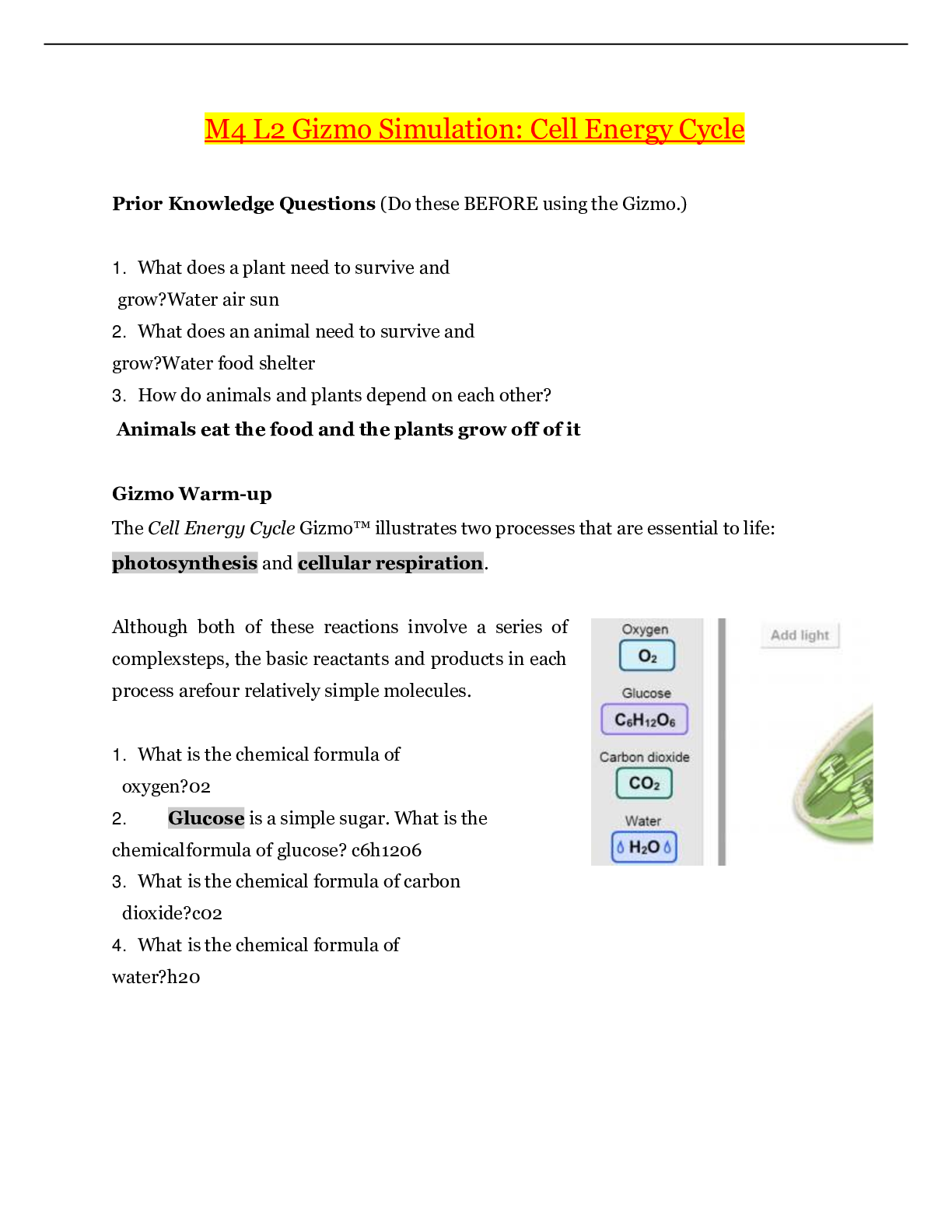
Name: Date: Student Exploration: Feel the Heat Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabulary: calori... meter, conductor, controlled experiment, dissolve, endothermic, exothermic, insulator, solute, solution, solvent, surroundings, system Gizmo Warm-up As you saw with the instant cold pack and the hand warmers, some reactions absorb energy while others release it. In the Feel the Heat Gizmo, you will explore these energy changes while making your own hot and cold packs. To begin, select the TEST POWDERS tab. Drag a bottle of NaC2H3O2 (sodium acetate) from the shelf. Using the sliders, decide how much water and powder to add. Note the beginning temperature, and then press Play ( ) to see what happens. 2. Experiment with different powders until you find one that produces the opposite effect. When a powder dissolves in water a solution is formed. The powder is the solute and the water is the solvent. Oftentimes energy changes accompany the formation of a solution. When added to water, some powders cause the resulting solution to get hot, while others make it cold. Some powders don’t produce a temperature change at all. When energy changes do occur, they can be put to good use. Activity A: Molecular view Get the Gizmo ready: ● Click Reset ( ). ● Drag a bottle of NaC2H3O2 from the shelf. Introduction: The reactions in this activity are performed within a calorimeter, an insulated device that keeps heat from escaping, enabling you to accurately record temperature changes. Question: Why does the temperature change when a powder is dissolved in water? 1. Observe: Turn on Show molecular view, and notice the water molecules. Set the Water volume to 100 mL and the Powder mass to 20 g, and then click Play. Click Pause ( ) after adding the powder. You should now see some sodium acetate in the water. A. What color represents the bonds between the particles of NaC2H3O2? B. Click Play. Watch the animation a few times. What happens to the NaC2H3O2 bonds? C. What happens to the bonds between water molecules? [Show More]
Last updated: 2 years ago
Preview 1 out of 6 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
May 23, 2021
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
May 23, 2021
Downloads
0
Views
128