eBook Process Gas Chromatography Advanced Design and Troubleshooting 1st Edition By Tony Waters
$ 29

ABO Ncle Actual 2024 Exam With 680 Answered Questions
$ 31.5
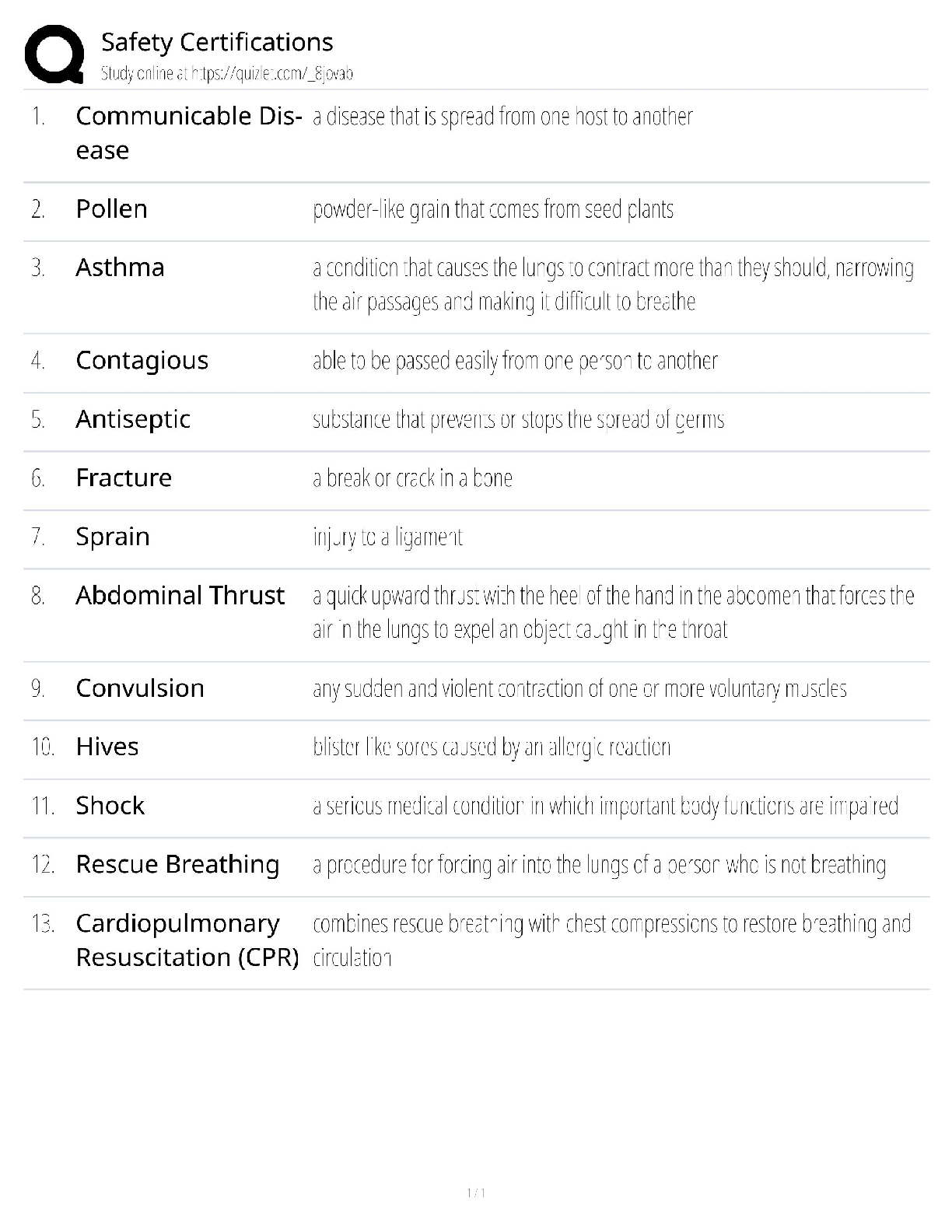
Safety Certifications / OSHA 10 & 30 Guide / 2025 Update / Score 100% Test Bank
$ 8.5

ACCT 212 Week 5 Quiz – 100% Correct Answers- Download To Score An A+
$ 15

CHEM-120 Unit 3 Lab: OL Lab 6: Solution Preparation: From Salt to Solution (GRADED)
$ 11

WGU C182 Introduction to IT Study Guide Already Passed
$ 5

PHILOSOPHY HESI PN EXIT V3 TEST BANK Complete Questions & Answers | Update 2023 - 2024
$ 14

Test bank For Data Visualization Exploring and Explaining with Data 2nd Edition By Jeffrey Camm, James Cochran, Michael Fry, Jeffrey Ohlmann
$ 30

2024 HESI LPN Anatomy and Physiology Guaranteed A+ Actual Questions and Answers, Complete 100%
$ 13

FDNY P-99 C OF F EXAM 2025
$ 10.5
.png)
PHSC 211 Chapter 16 Quiz (Fall 2022)– Liberty University (A grade) | Elements of Earth Science
$ 7

CHEM-120 Unit 4 Lab: OL Lab 8: Acids and Bases (GRADED)
$ 9

Pas-07-Statement-Of-Cash-Flows
$ 30

CHOLECYSTITIS NCLEX QUESTIONS AND VERIFIED ANSWERS WITH NGN LATEST 2023-2024
$ 16
.png)
PHSC 211 Chapter 6 Quiz (Fall 2022) – Liberty University (A grade) | Elements of Earth Science
$ 8

APPRENTICE LINEMAN GENERAL KNOWLEDGE 2024/2025 STUDY QUESTIONS WITH VERIFIED ANSWERS GUARANTEED PASS | RATED A+
$ 12.5

[eBook] [PDF] Fundamentals of Anatomy and Physiology of Speech, Language, and Hearing 1st Edition By Glen Tellis ,Hunter Manasco
$ 30
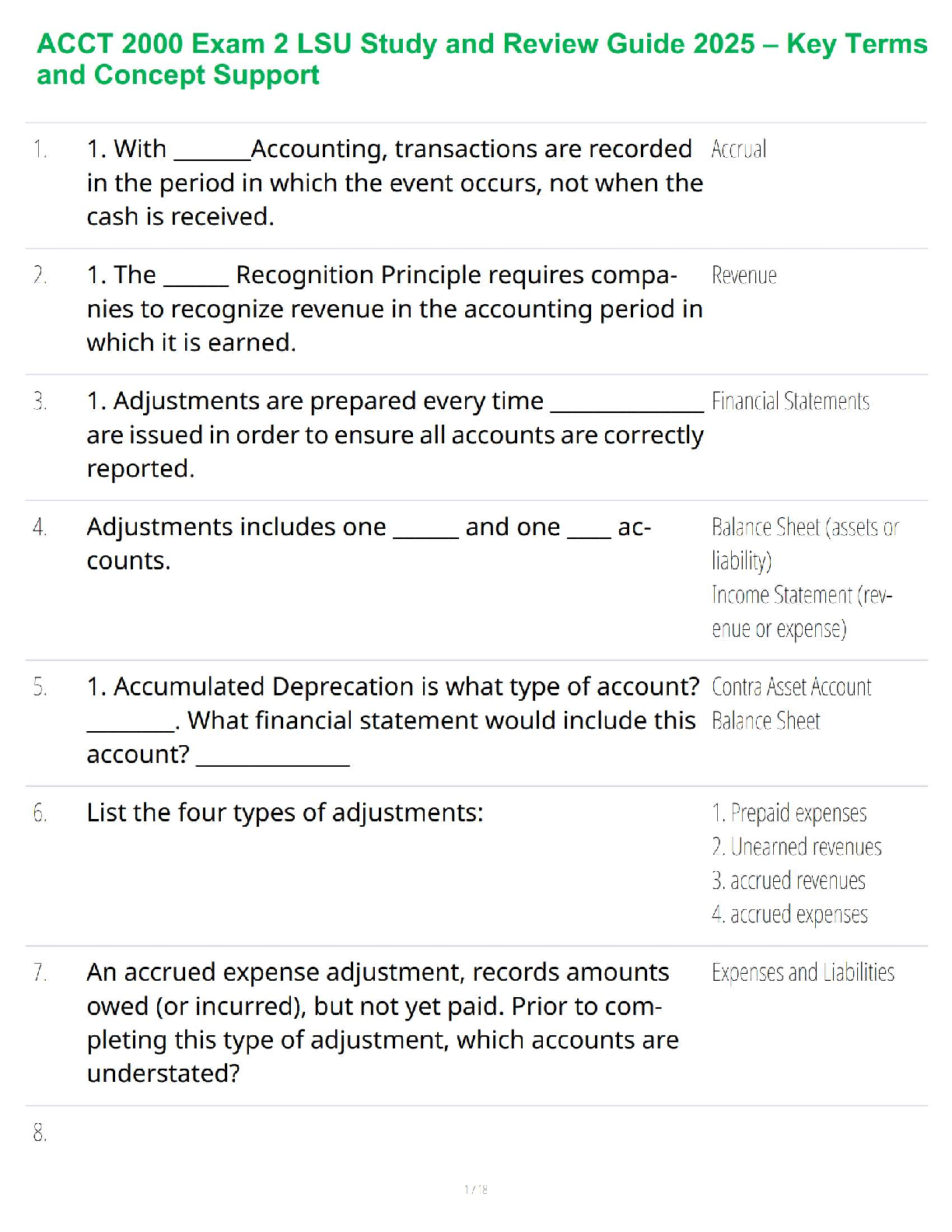
ACCT 2000 Exam 2 LSU Study and Review Guide 2025 – Key Terms and Concept Support
$ 14
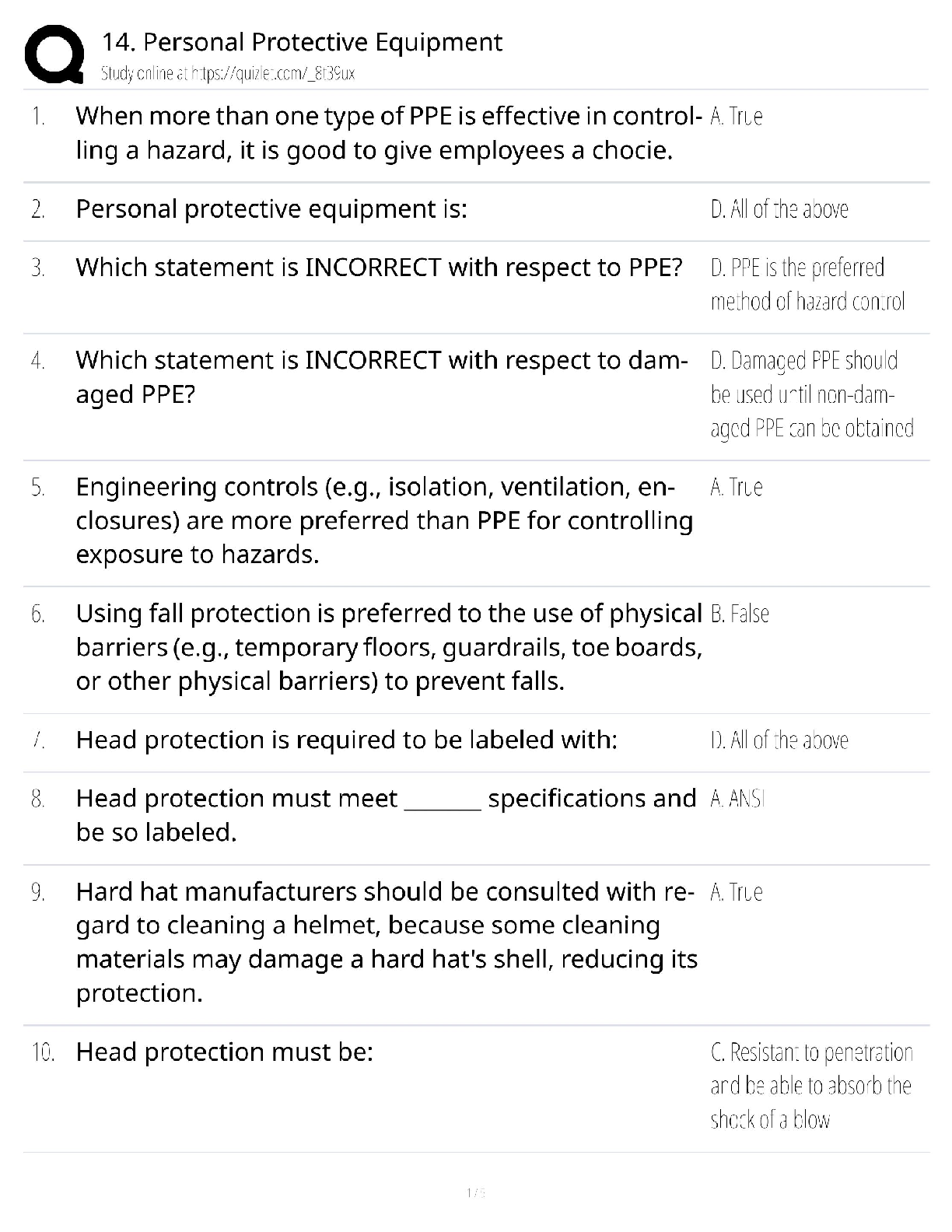
Personal Protective Equipment Test Bank / OSHA Safety Certification / 2025 Update / Score 100%
$ 19.5

TMF 12 TEXAS FIRE ALARM TECHNICAL EXAM PREPARATION VERSION 1&2 2025/2026
$ 10

Chapter 06Environmental Conservation: Forests, Grasslands, Parks and Nature Preserves
$ 13

✅ Fire Department of New York (FDNY) – Z51 Test 2025/2026
$ 19.5

A/AS Level Question Bank Topic: 3.2.1
$ 3

Counterbalance & Rough Terrain Forklift – Operator Certification Exam – Actual Questions with Verified Correct Answers
$ 8
ATI MED SURG STUDY GUIDE-NUR1234.png)
ATI MED SURG STUDY GUIDE-NUR1234
$ 14

PHSC 211 Chapter 7 Quiz (Fall 2022)– Liberty University (A grade) | Elements of Earth Science
$ 8

REVISION SCIENCE TO EXCEL IN A-LEVEL PHYSICS A-levelPHYSICS7407/2 Paper 2
$ 12

Spatial Inequalities and Policies in South Africa: Place-based or People-centred?
$ 20

NICET FIRE ALARM LEVEL II EXAM PREPARATION 2025/2026
$ 10
.png)
PHSC 211 Chapter 3 Quiz (Fall 2022)– Liberty University (A grade) | Elements of Earth Science
$ 8

eBook [PDF] The Special Liveliness of Hooks in Popular Music and Beyond 1st Edition By Steven G. Smith
$ 29

Harvard University {Data analytics Quizzes , BUSINESS CORE} questions and answer 100 % correct | kindly contact +1 (681) 229 7738 for any academic assistance.
$ 44
.png)
PHSC 211 Chapter 8 Quiz (Fall 2022)– Liberty University (A grade) | Elements of Earth Science
$ 7

Solution manual Legal research, analysis, and writing, 5th edition By putman, albright, chapters 1 19
$ 14

📘 OSHA 10-Hour Test Questions 2025 ✅
$ 20

PHSC 211 Chapter 13 Quiz (Fall 2022)– Liberty University (A grade) | Elements of Earth Science
$ 7

CHEM-120 Unit 4 Lab: OL Lab 8: Acids and Bases (GRADED)
$ 5.5

NICET FIRE ALARM LEVEL I EXAM PREPARATION 2025/2026
$ 10

SOCIOLOGY 2266 FINAL EXAM NOTES CRIMINAL BEHAVIOR
$ 14

NCLEX RN COMPREHENSIVE EXIT EXAMS WITH NGN LATEST MULTIPLE VERSIONS COMBINED LATEST UPDATE 2023-2024
$ 30.5

ATI MENTAL HEALTH RETAKE GUIDE
$ 17.5

ACCT 2020 Principles of Accounting Midterms (Qns & Ans) 2025 - WGU
$ 12
.png)
To Kill a Mockingbird Final Test Review Already Passed
$ 10
.png)
ACCT 212 WEEK 1 QUIZ (awarded points 100) | DeVry University
$ 10

BSW License Exam Study Guide with complete solutions
$ 10

ENVS 1126 Final Exam | Complete Solutions (Answered)
$ 16

MKT 3210 EXAM 2 VOCAB STUDY GUIDE
$ 5

NSG 280 Practice Questions (Questions Only)2020
.png)