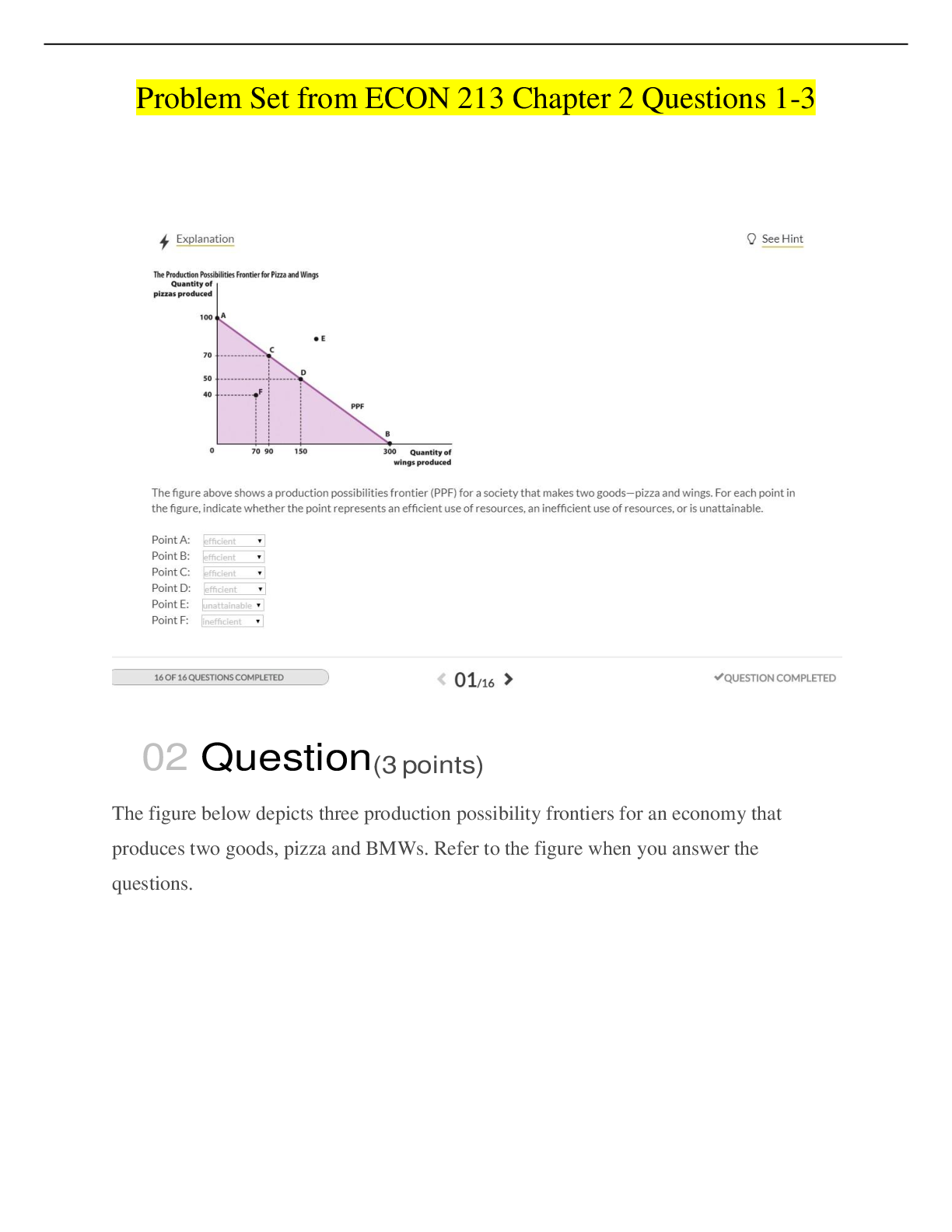
Question type: Multiple choice
1) Which of the following compounds contains polar covalent bonds?
A) CS2
B) LiF
C) F2
D) CH3F
E) None of these choices.
2) Which molecule does not have a dipole moment?
...
Question type: Multiple choice
1) Which of the following compounds contains polar covalent bonds?
A) CS2
B) LiF
C) F2
D) CH3F
E) None of these choices.
2) Which molecule does not have a dipole moment?
3) Of the following solvents which one does not have a zero dipole moment?
A) Pentane
B) Cyclohexane
C) Diethyl ether
D) Cyclopentane
E) None of these choices.
4) Which molecule has a zero dipole moment?
A) CH3Cl
B) CH2Cl2
C) CHCl3
D) CCl4
E) None of these choices.
5) Which molecule would you expect to have no dipole moment (i.e., = 0 D) ? A) CHF3
F H
B) HF
C) :NF3
F F
D)H H
E) CH2F2
6) Which molecule has a dipole moment greater than zero?
F F
A)H H
F H
B)F H
F H
C) HF
D) More than one of these choices.
E) None of these choices.
7) Which of the following would have no net dipole moment ( = 0 D) ?
A) CBr4
B) cis-1,2-Dibromoethene
C) trans:-1,2-Dibromoethene
D) 1,1-Dibromoethene
E) More than one of these choices.
8) For a molecule to possess a dipole moment, which following condition is necessary but not sufficient?
A) three or more atoms in the molecule
B) presence of one or more polar bonds
C) a non-linear structure
D) presence of oxygen or fluorine
E) absence of a carbon-carbon double or triple bond
9) Which molecule has a zero dipole moment?
A) SO2
B) CO2
C) CO
D) CHCl3
E) None of these choices.
10) Which molecule has a zero dipole moment?
A) CO2
B) CH4
C) CH3CH3
D)
E) All of these choices.
11) Which molecule has a dipole moment of zero?
A) CHCl3
B) CH2Cl2
C) ClHC=CH2
D) trans-ClHC=CHCl
E) None of these choices.
12) Which molecule would have a dipole moment greater than zero?
A) BeCl2
B) BCl3
C) CO2
D) H2O
E) CCl4
13) A non-zero dipole moment is exhibited by:
A) SO2
B) CO2
C) CCl4
D) BF3
Cl Cl
E) Cl Cl
14) Of the following common organic solvents which one is predicted to have the smallest dipole moment?
A) Chloroform, CHCl3
B) Acetone, (CH3) 2CO
C) Dimethylsulfoxide, (CH3) 2SO
D) Acetonitrile, CH3CN
E) Methanol, CH3OH
15) Which molecule(s) has/have dipole moment(s) equal to zero?
16) What alkyl groups make up the following ether?
A) ethyl and phenyl
B) propyl and benzyl
C) ethyl and benzyl
D) propyl and phenyl
E) None of these choices.
17) What alkyl groups make up the following ketone?O
A) Phenyl, pentyl
B) Hexyl, phenyl
C) Benzyl, hexyl
D) Benzyl, heptyl
E) None of these
18) What alkyl groups make up the following ether? O
A) Isobutyl and methyl
B) Methyl and butyl
C) Ethyl and isopropyl
D) Methyl and sec-butyl
E) None of these choices.
19) What alkyl groups make up the following ether?
A) isobutyl and propyl
B) propyl and butyl
C) ethyl and isopropyl
D) propyl and sec-butyl
E) None of these choices.
20) What alkyl group is attached to the oxygen in the following ester?O
A) ethyl
B) propyl
C) sec-propyl
D) isopropyl
E) None of these choices.
21) What alkyl groups make up the following 3o amine?
A) sec-butyl, ethyl, propyl
B) isobutyl, isopropyl, ethyl
C) sec-butyl, ethyl, isopropyl
D) butyl, ethyl, propyl
E) None of these choices.
22) What alkyl groups are attached to the benzene ring in the following example?
H3CH2CH2C
A) ethyl, butyl
B) ethyl, isobutyl
C) propyl, sec-butyl
D) propyl, butyl
E) None of these choices.
23) What common group is attached to both the ether and 3o amine in the following molecule?
OCH2C6H5
NBn
[Show More]