Chemistry > GIZMOS > GIZMOS. CHEMISTRY Periodic Trends Gizmo Questions:Consisting of Prior Knowledge Questions, Gizmo War (All)
GIZMOS. CHEMISTRY Periodic Trends Gizmo Questions:Consisting of Prior Knowledge Questions, Gizmo Warm-up, Activity A: Atomic radius, Activity B: Removing and adding electrons, Activity C: Periodic trends
Document Content and Description Below
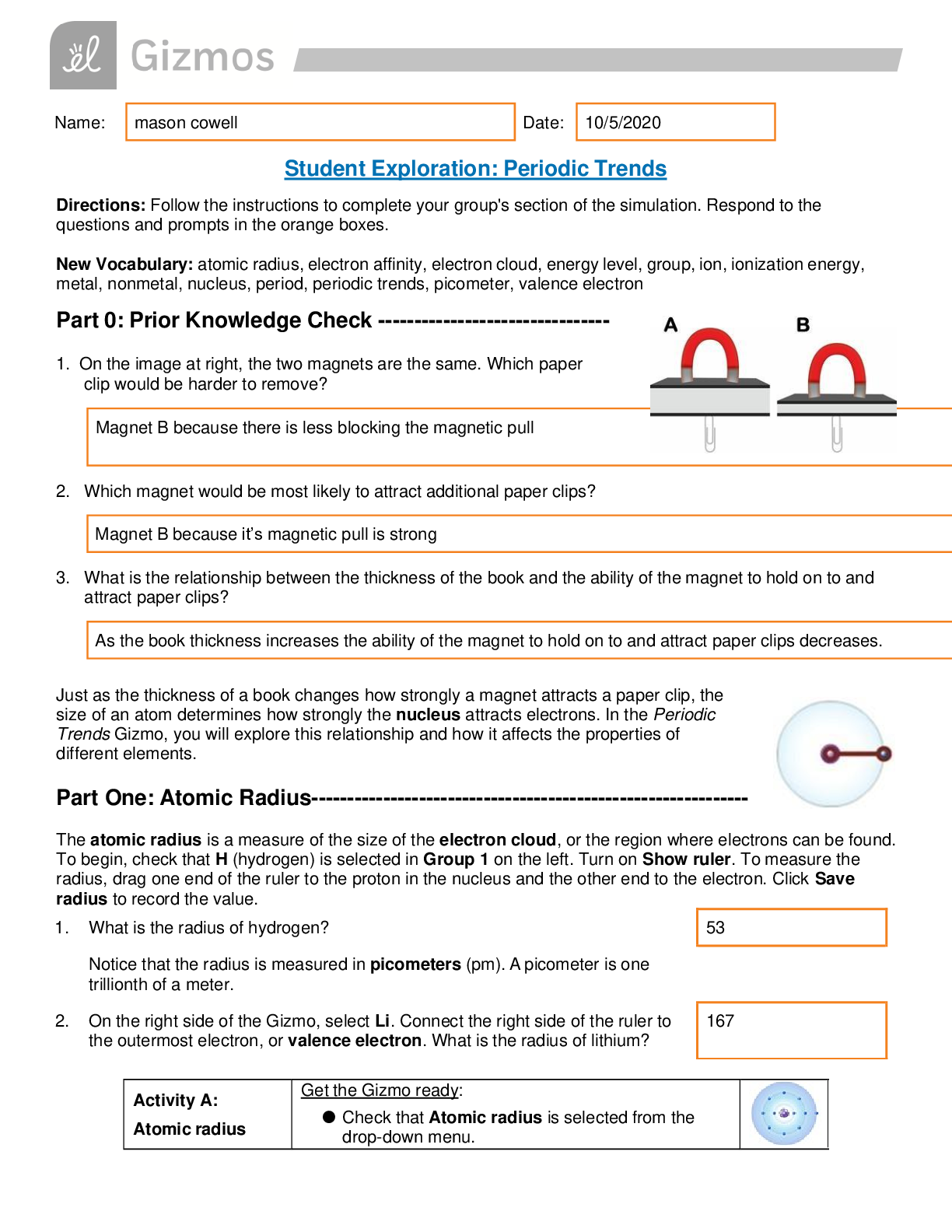
Question: What factors affect the radius of an atom? 1. Predict: How do you think the radius of an atom will change as you move down a group (vertical column) in the periodic table? 2. Collect data... : Use the ruler to measure the atomic radii of the group 1 elements. As you do so, count the energy levels (shown as rings of electrons) in each atom. Record in the table. Element H Li Na K Rb Cs Fr Number of energy levels 1 2 3 4 5 6 Atomic radius (pm) 53 167 190 243 265 298 3. Observe: What happens to the radius as you move down group 1? It gets bigger as more energy levels are added. 4. Explore: Turn off Show ruler. Select Li, and then select Be. Observe the radii of the elements in group 2. Then look at other groups. What pattern do you see? The radius of each group gets smaller than the group before it. 5. Draw a conclusion: In general, what is the effect of the number of energy levels on the radius of an atom? The more energy levels an atom has the larger the radius 6. Predict: How do you think the radius of an atom will change as you move across a period (horizontal row) in the periodic table? I think that the radius will continue to get smaller. 7. Collect data: Beginning with Na, record the number of energy levels, number of protons, and atomic radius for each element in period 3. Element Na M [Show More]
Last updated: 2 years ago
Preview 1 out of 9 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 15, 2021
Number of pages
9
Written in
Additional information
This document has been written for:
Uploaded
Apr 15, 2021
Downloads
0
Views
153

.png)








.png)