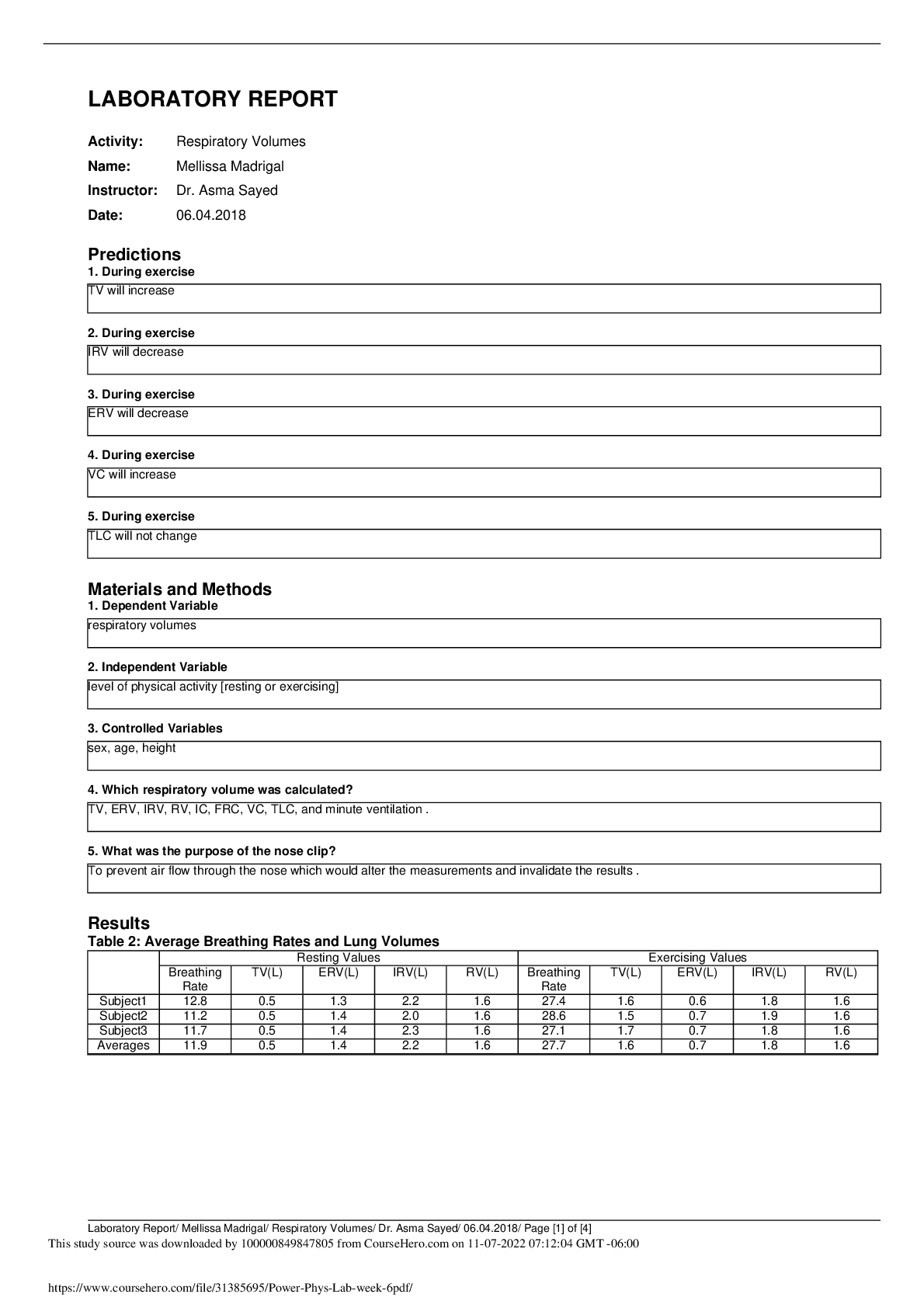
Candies Woods
5/31/2020
Energy Forms & Changes Virtual Lab
Introduction: You will use the PhET simulation Energy
Forms & Changes to predict, experiment, discover and
interpret the meaning of the material property kn
...
Candies Woods
5/31/2020
Energy Forms & Changes Virtual Lab
Introduction: You will use the PhET simulation Energy
Forms & Changes to predict, experiment, discover and
interpret the meaning of the material property known as
Specific Heat Capacity.
Warm-Up:
1. Describe what the following terms mean
Temperature – the measure of the average kinetic energy per molecule of a substance.
Heat – the energy that flows from one object to another due to a temperature difference between them.
2. Using your definition for temperature above, explain how a thermometer works to provide a visual
representation of the temperature
Ans: Many thermometers are thin glass filled with a liquid. A change in temperature causes a small
change in the volume of the liquid. Most thermometers measure temperature by the by means of
expansion or contraction of the liquid. When the liquid expands in the tube of the thermometer it is
magnified, there is a change in height and that is what we see.
3. Thermometers in the past were filled with mercury but most are now filled with alcohol
instead due to safety reasons. Why do you think alcohol is used instead or water?
Ans: Alcohol is used instead of water because it boasts a higher thermal expansion. In
addition, alcohol has a very low freezing point of -114°C (-173.5 °F) while water freezes at
0°C (32 °F). Water would hinder the use of a thermometer when it's freezing outside.
4. The simulator allows you to add the same amount of heat energy to different materials e.g. water, oil,
iron, brick. If you can measure the temperature of these materials as you are heating them would you
predict that each will have the same temperature changes? Explain.
Ans: I think the temperature would change for all of them just at a different rate. The amount of heat needed to
raise the temperature for each material is different. They all have different specific heat capacity. For example,
the oil would heat up faster than water because it has a lower heat capacity
[Show More]






.png)










.png)





.png)


