Enthalpy of Formation Using Hess’s Law
Student Name
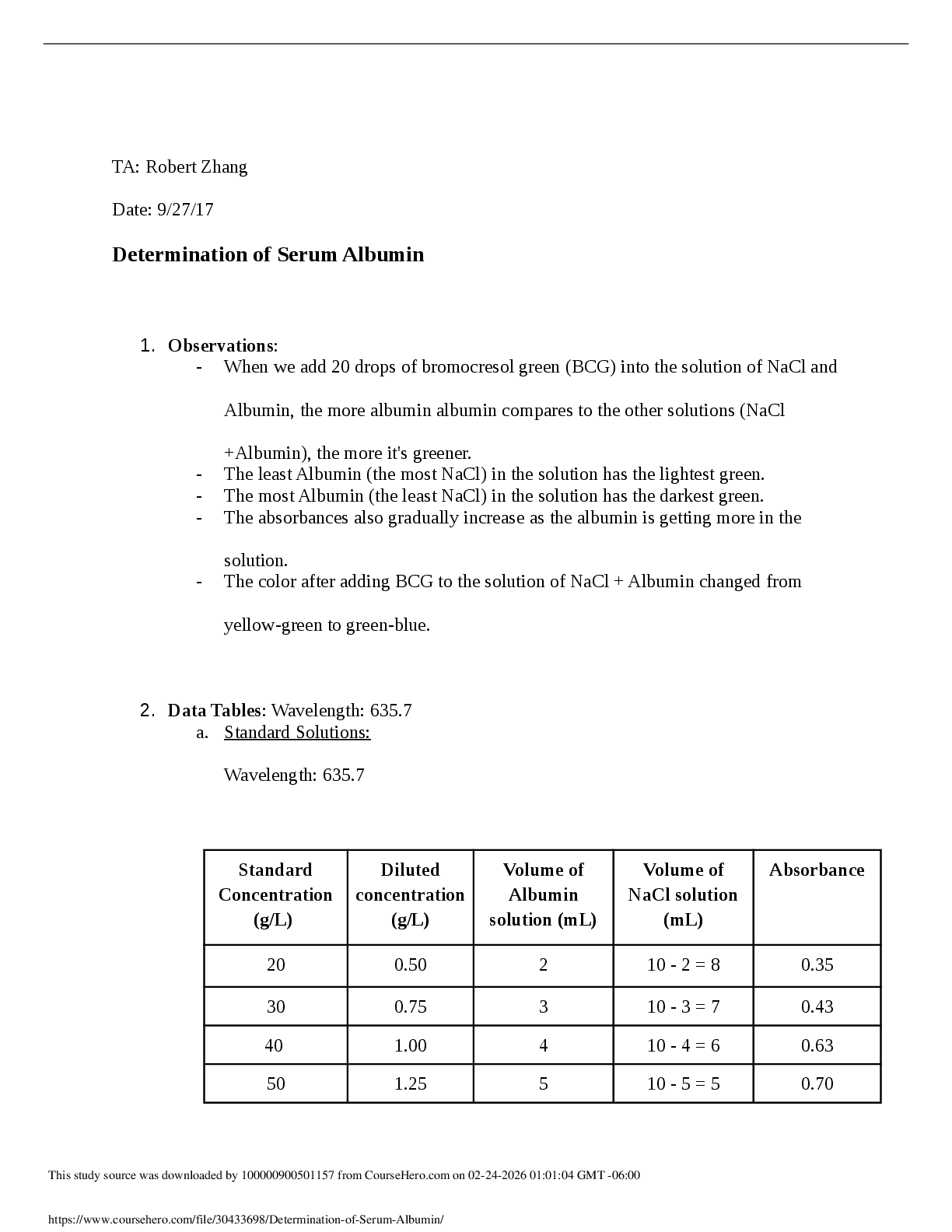
June 26, 2020Chemical Hazards:
- Wear safety goggles, chemical apron and gloves at all times
- Keep both magnesium and magnesium oxide away from any heat or
flame
...
Enthalpy of Formation Using Hess’s Law
Student Name
June 26, 2020Chemical Hazards:
- Wear safety goggles, chemical apron and gloves at all times
- Keep both magnesium and magnesium oxide away from any heat or
flame source
- Keep chemicals away from food storage locations to avoid accidental
ingestion.
- Do not eat, drink, or chew gum while performing lab
- Wash hands with soap and water before and after performing lab.
Goals:
- Determination of ΔHrxn Mg and HCl for Activity 2,
- Will measure the temperature change and calculate the heat
generated during this reaction
- Determination of ΔH
rxn
MgO and HCl for Activity 3
- Will react magnesium oxide with hydrochloric acid, this reaction is the
reverse of Activity 2 so the ΔH2 will have the opposite sign when
calculations are performed.
Introduction:
In this experiment I will determine the enthalpy of formation of
magnesium oxide using Hess’ law. The instrument I will use in this lab is the
calorimeter, which is an insulated apparatus designed to create a closed
system by preventing heat from flowing in or out. The equations of chemical
reactions I’ll perform are
Equation 1: Mg(s) + 2 H+(aq) → Mg2+(aq) + H2 (g); Equation 2: Mg2+(aq) +
H2O(l) → MgO(s) + 2 H+(aq) ; and Equation 3: H2(g) + 1⁄2 O2(g) → H2O(l). I will
find Hf because it is the standard enthalpy of formation, and it’s the heat
associated with the formation of one mole of a compound at one atmosphere
of pressure at 25 degree Celsius. Due to Hess’s law, a reaction can be
performed in steps and the sum of the ΔHrxn for each of those steps is equal
to the ΔHf for the compound in question (in this case, magnesium oxide). So,
in other words, we are able to overcome the obstacle of the three members
in the equations (H1 + H2 + H3)
[Show More]