Fractional Precipitation
Can one type of cation be removed from an aqueous mixture of multiple cations by
precipitation? Why? In both industry and research, there are often times when one particular
component of a mix
...
Fractional Precipitation
Can one type of cation be removed from an aqueous mixture of multiple cations by
precipitation? Why? In both industry and research, there are often times when one particular
component of a mixture needs to be separated froma solution. Maybe it is a rare metal that is
dissolved in a mixture of minerals. Maybe it is aparticular protein from lysed plant cells. If thedesired
component is volatile, distillation could be used. But if the goal is to separate ions in solution,
fractional precipitation is preferred.
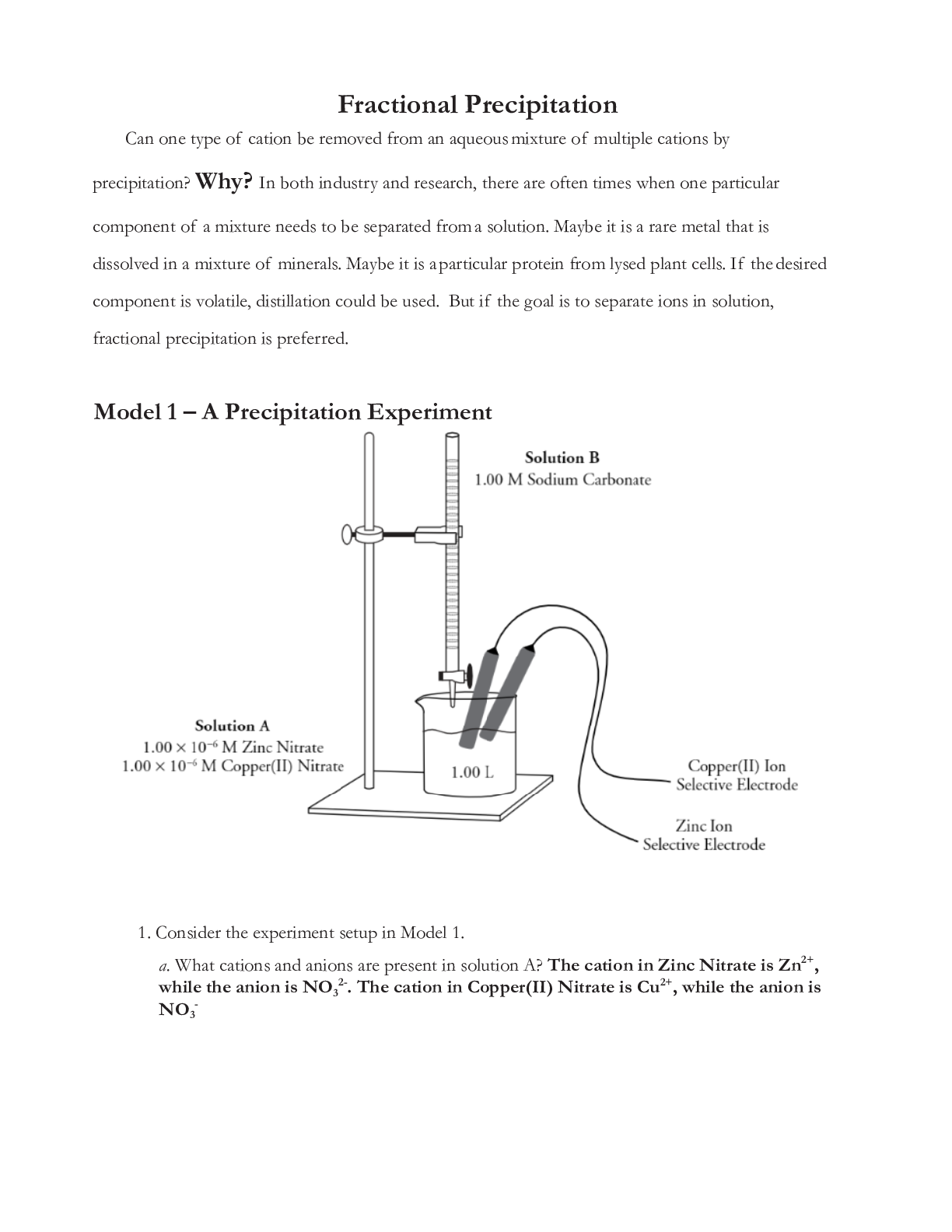
Model 1 – A Precipitation Experiment
1. Consider the experiment setup in Model 1.
a. What cations and anions are present in solution A? The cation in Zinc Nitrate is Zn2+,
while the anion is NO32-. The cation in Copper(II) Nitrate is Cu2+, while the anion is
NO3-b.
What is the starting molar concentration of solution A for both zinc and copper(II) ions?
Zinc ions starting concentration is 1.00 x 10-6 and Copper(II) ions starting
concentration is 1.00 x 10-6 as well.
c. Identify the cations and anions present in solution B. The cation in Sodium carbonate
is Na+, while the anion is CO3-
d. What is the concentration of carbonate ions insolution B? 1.00 M
Fractional Precipitation 1
2. When solution A and solution B mix, there is potential for two precipitates to form. Write
double replacement reactions to show the formation of the two precipitates.
Zn(s) + Cu(NO3)2(aq) → Zn(NO3)2(aq) + Cu(s)z
3. In the experiment setup in Model 1, how will the concentration of zinc and copper(II) ions in
solution A be recorded during the experiment? A pH indicator will record the
concentration of the Zinc andCopper(II) ions
[Show More]





















.png)

