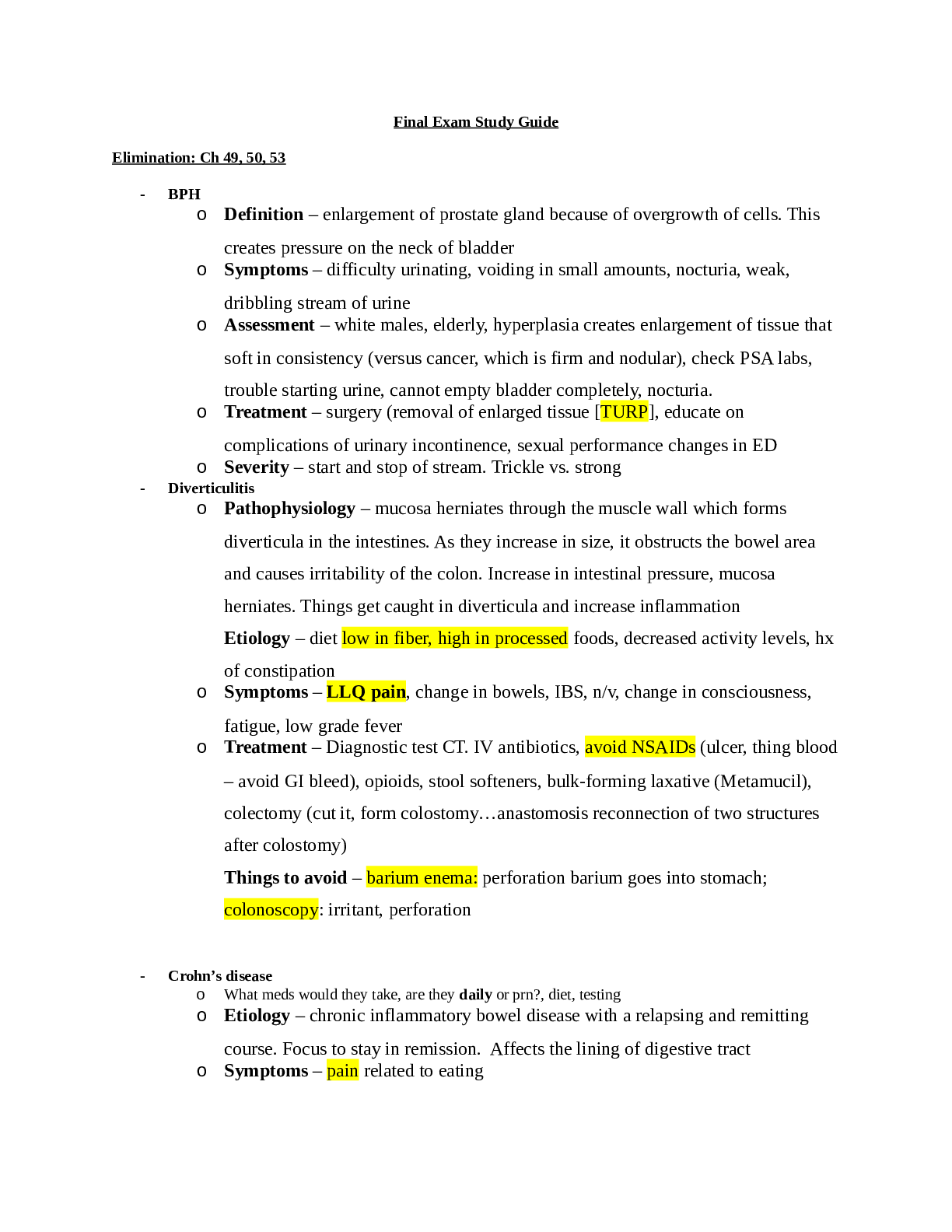
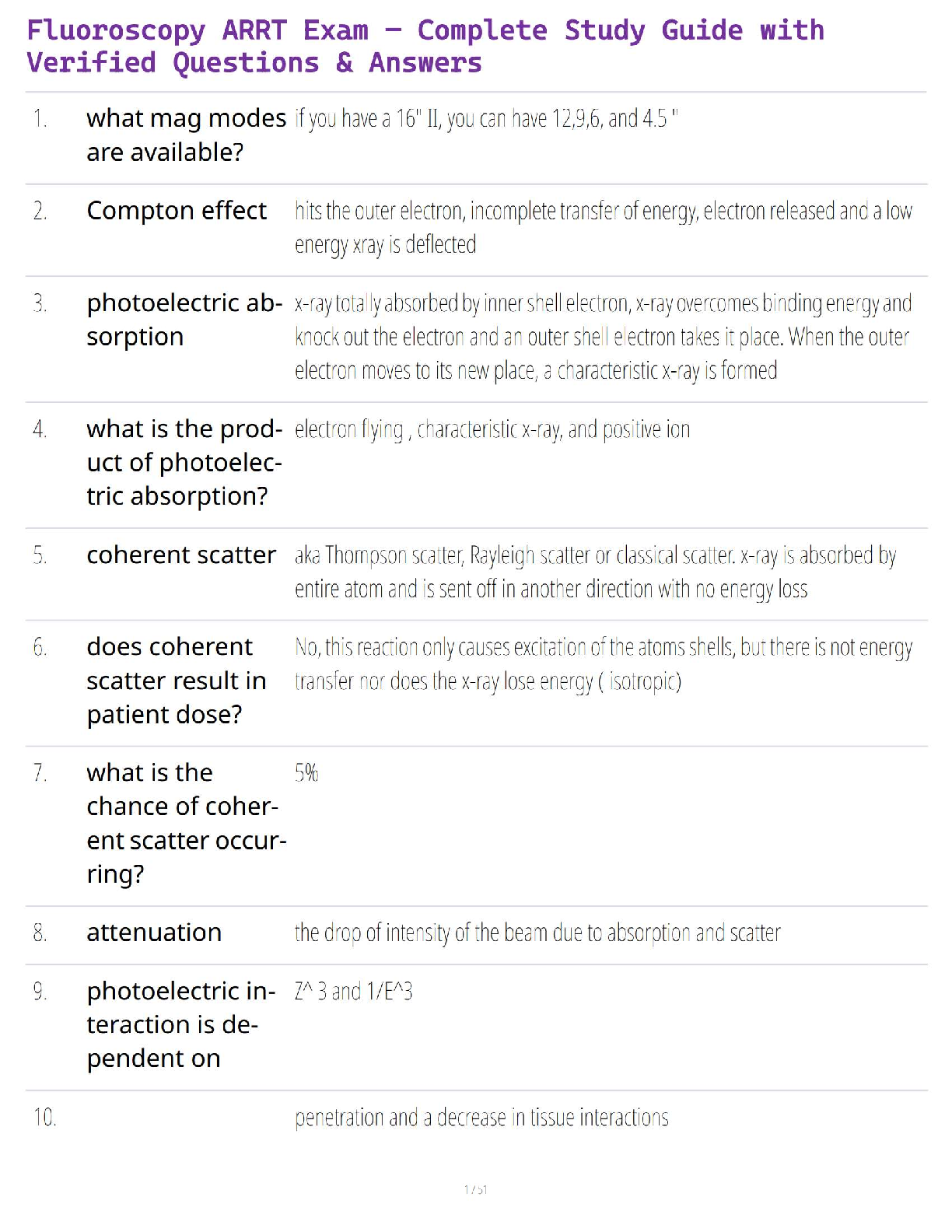
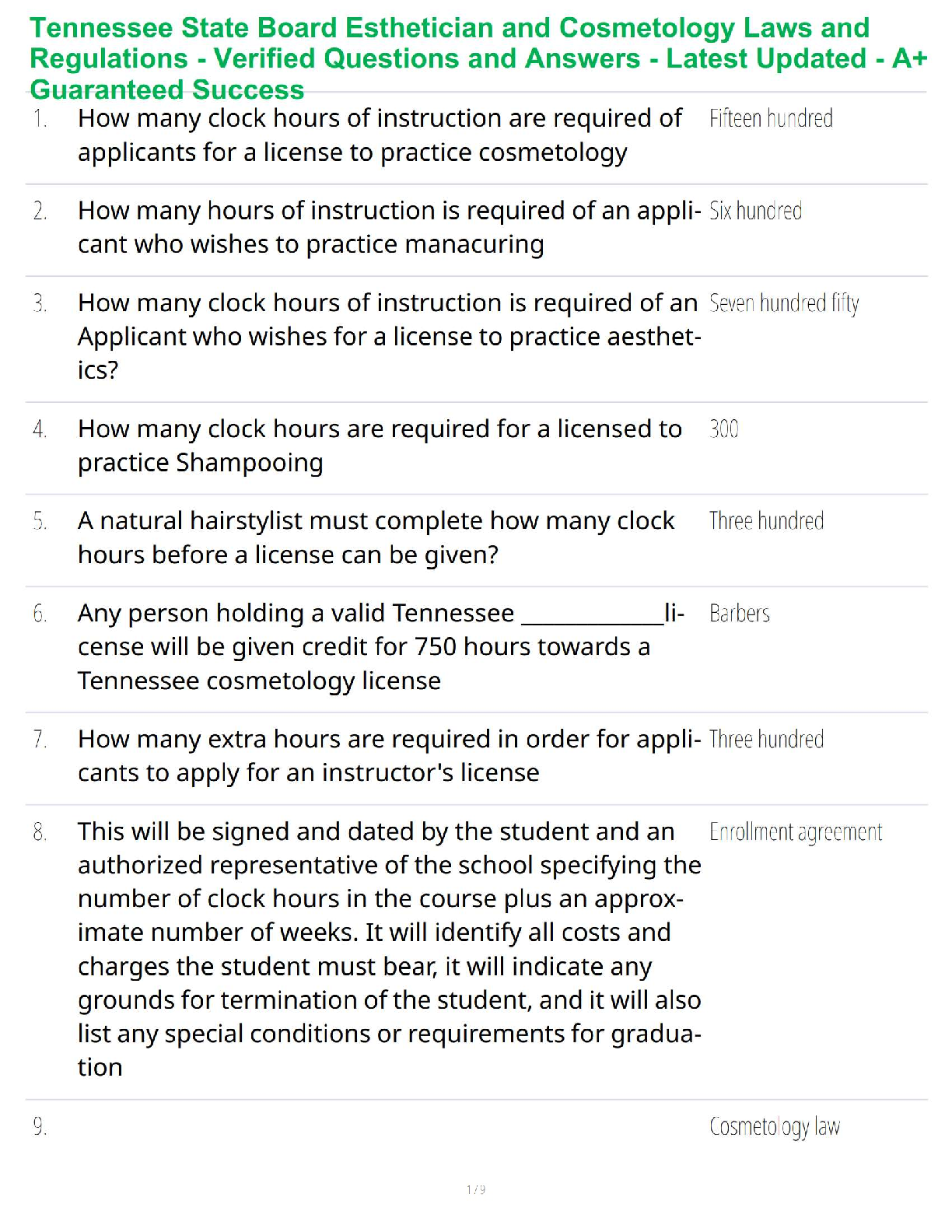
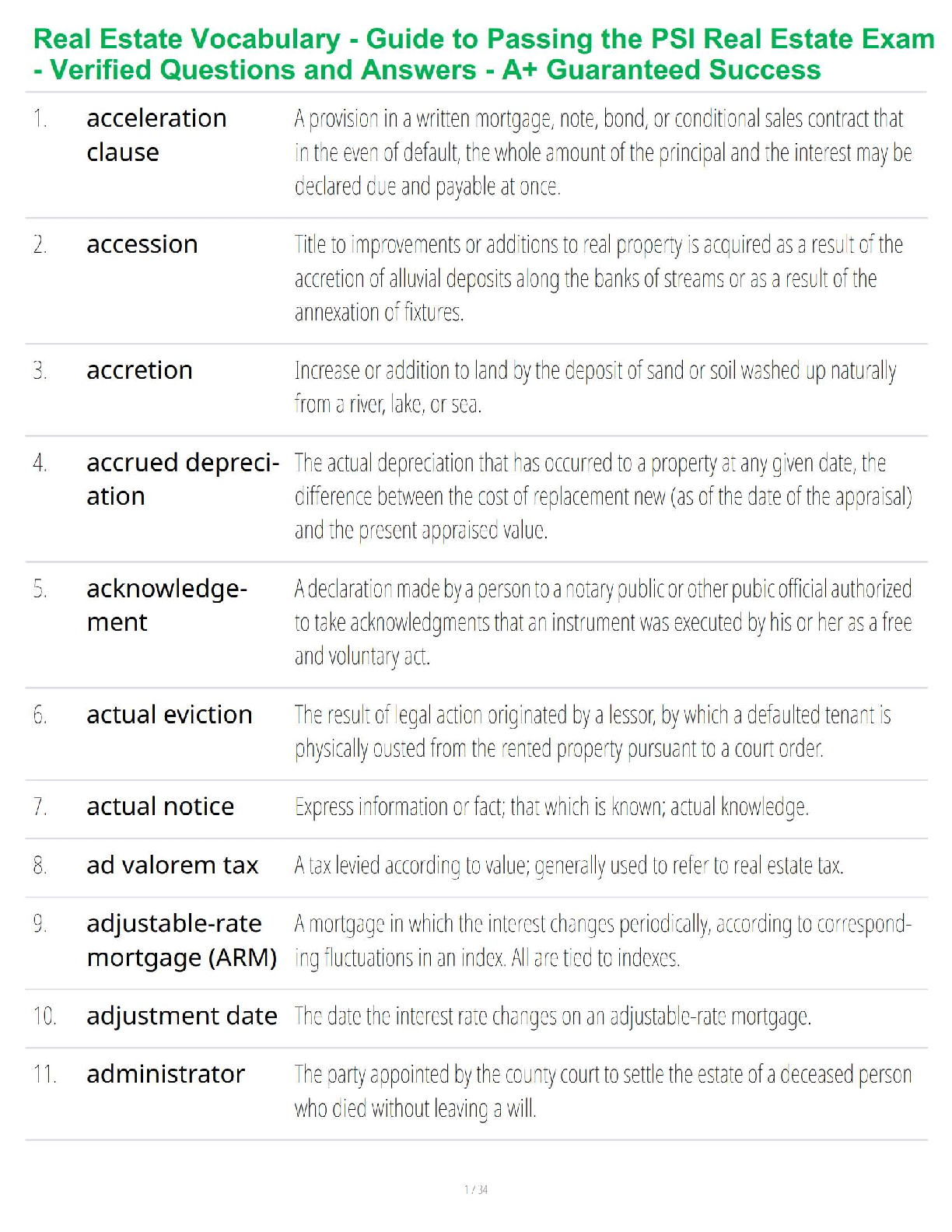
Chemistry Journal
8.5 Acid and Base Calculations
Driving Question: How do hydronium and hydroxide ion concentrations affect pH, and how
are these concentrations used in stoichiometric calculations?
Key Ideas and
Ter
...
Chemistry Journal
8.5 Acid and Base Calculations
Driving Question: How do hydronium and hydroxide ion concentrations affect pH, and how
are these concentrations used in stoichiometric calculations?
Key Ideas and
Terms
Notes
FQ: What are the amphoteric properties of water?
What is an
amphoteric
substance?
a substance that acts as an acid or a base by both receiving and
donating hydrogen ions
Video: Amphoteric Substance
How does
water act like
an acid and a
base?
Water is what is known as an amphoteric substance. It can act as an acid or
a base by both receiving and donating hydrogen ions. It can be used in
chemical reactions to split some other molecules, called dissociation. For
example, hydrogen chloride dissociates almost completely in water to form
hydrochloric acid. The hydrogen ion in the HCl molecule forms a
hydronium ion with a water molecule and a chlorine ion.
How do water
molecules react
with one another?
Water molecules can react with each other in a sample of pure water.
One water molecule, acting as an acid, donates a hydrogen ion to
another water molecule, acting as a base. This reaction forms
hydroxide and hydronium ions, which can also react together to reform the water molecules. Water has an ionization constant to
represent the number of hydronium and hydroxide ions present in a
sample of water at one time.
What is the
ionization constant
of water?
Water has an ionization constant to represent the number of
hydronium and hydroxide ions present in a sample of water at one
time.
If the pH of a
solution is 12,
The product of the molarities for hydronium and hydroxide molecules
always equals 1.0 x 10−14 M. For example, at a pH of 12, the hydronium
This study source was downloaded by 100000822472680 from CourseHero.com on 05-27-2021 20:31:44 GMT -05:00
https://www.coursehero.com/file/41061317/08-05-journaldoc/
This study resource was
shared via
[Show More]