NR 602 Array Midterm Study Guide (All Correct Study Guide) Download to Score ANR 602 Array Midterm Study Guide (All Correct Study Guide)
Signs of pregnancy
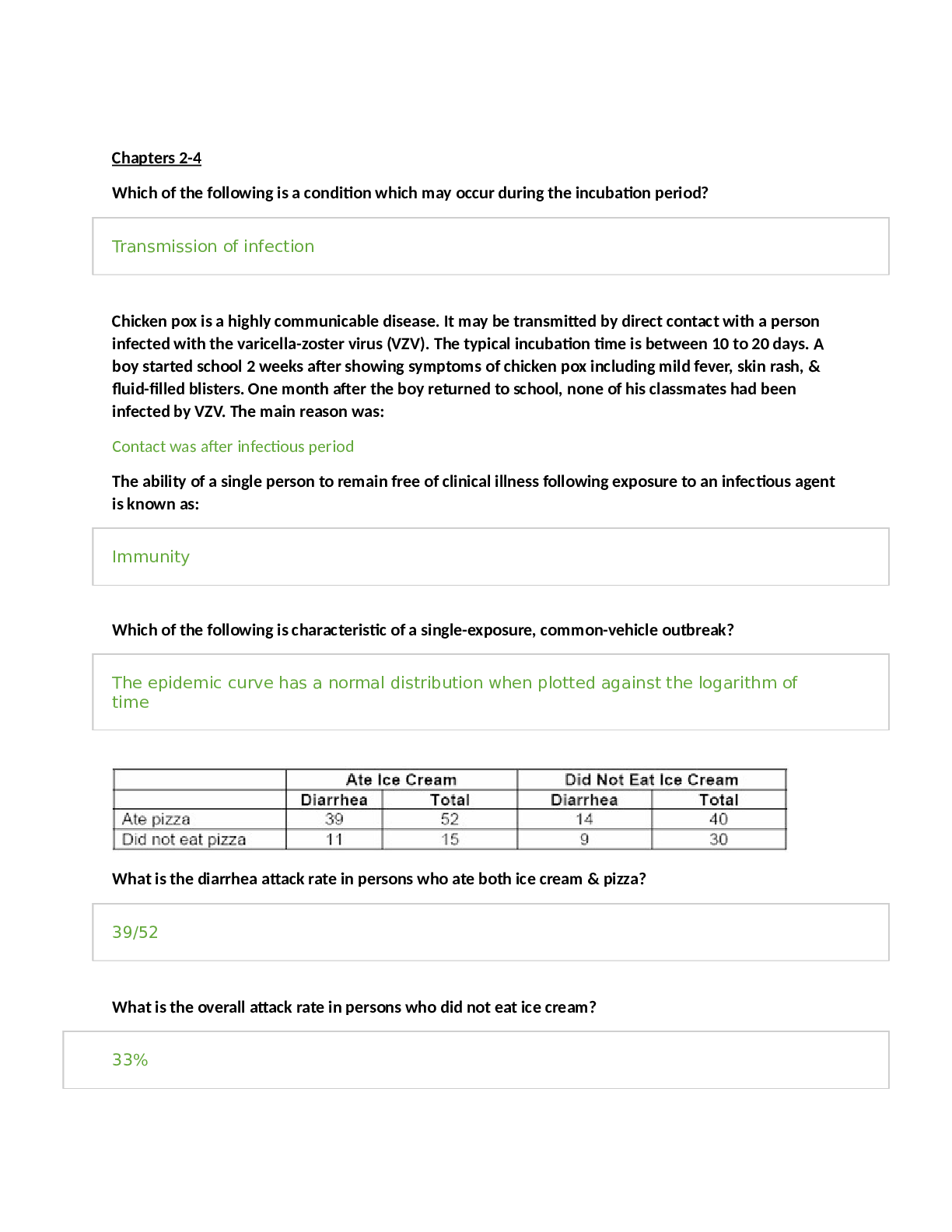
presumptive (subjective signs) Amenorrhea, nausea, vomiti
...
NR 602 Array Midterm Study Guide (All Correct Study Guide) Download to Score ANR 602 Array Midterm Study Guide (All Correct Study Guide)
Signs of pregnancy
presumptive (subjective signs) Amenorrhea, nausea, vomiting, increased urinary frequency, excessive fatigue, breast tenderness, quickening at 18–20 weeks
probable (objective signs) Goodell sign (softening of cervix) Chadwick sign (cervix is blue/purple)
Hegar’s sign (softening of lower uterine segment) Uterine enlargement
Braxton Hicks contractions (may be palpated by 28 weeks)
Uterine soufflé (soft blowing sound due to blood pulsating through the placenta) Integumentary pigment changes
Ballottement, fetal outline definable, positive pregnancy test (could be hydatidiform mole, choriocarcinoma, increased pituitary gonadotropins at menopause)
positive (diagnostic signs) Fetal heart rate auscultated by fetoscope at 17–20 weeks or by Doppler at 10–12 weeks
Palpable fetal outline and fetal movement after 20 weeks
Visualization of fetus with cardiac activity by ultrasound (fetal parts visible by 8 weeks)
Pregnancy and fundal height measurement
Signs of pregnancy (presumptive, probable, positive)
Pregnancy and fundal height measurement As pregnancy progresses, the fundus rises out of the pelvis (Figure 29-1). At 12 weeks’ gestation, the fundus is located at the level of the symphysis pubis. By week 16, it rises to midway between symphysis pubis and the umbilicus. By 20 weeks’ gestation, the fundus is typically at the same height as the umbilicus. Until term, the fundus enlarges approximately 1 cm per week. As the time for birth approaches, the fundal height drops slightly. This process, which is commonly called lightening, occurs for a woman who is a primigravida around 38 weeks’ gestation but may not occur for the woman who is a multigravida until she goes into labor
Naegele’s rule
Add seven days to the first day of your LMP and then subtract three months. For example, if your LMP was November 1, 2017: Add seven days (November 8, 2017). Subtract three months (August 8, 2017).
The EDD is calculated by adding seven days to the first day of the last menstrual period, subtracting three months and adding one year.
This formula is known as Naegele's Rule. For example, if the patient's last menstrual period, LMP, was on August 10, 2019, the EDD would be calculated as follows. LMP equals August 10, 2019 plus seven days. August 17, 2019, minus three months. May 17, 2019 plus one year and that equals May 17, 2020.
Hematological changes during pregnancy
During pregnancy, the heart is displaced upward and to the left within the chest cavity by the gravid uterus’s pressure on the diaphragm. As pregnancy progresses, the risk for inferior vena cava and aortic compression leading to supine hypotension increases when the woman lies in a supine position. To avoid hypotension and potential syncope, the woman should be advised to lie in a left lateral position. Hemodynamic changes and anatomic changes also may alter vital signs in the pregnant woman (Table 29-2).
Cardiac output in pregnancy increases by 30% to 50% over that in women who are not pregnant (Blackburn, 2013; Ouziunian & Elkayam, 2012). This increase peaks in the early third trimester and is maintained until birth. Half of the total increase in cardiac output, however, occurs by the eighth week of pregnancy (Blackburn, 2013). Therefore, women with cardiac disease may become symptomatic during the first trimester. Stroke volume is also increased during pregnancy by 20% to 30%. These increases in cardiac output and stroke volume allow for the 30% increase in oxygen consumption observed during pregnancy.
TABLE 29-2 Vital Sign Changes in Pregnancy
Vital Sign Changes in Pregnancy Measurement Alterations in Pregnancy
Heart rate and heart sounds Volume of the first heart sound may be increased with splitting. Third heart sound may be detected.
Systolic murmurs may be detected. Increases by 15–20 beats/min by 32 weeks’ gestation. Palpate the maternal pulse when auscultating the fetal heart rate to be able to distinguish between the two.
Respiratory rate Increases by 1–2 breaths/min None
BP First trimester: same as prepregnancy values
Second trimester: systolic BP decreases by 2–8 mm Hg and diastolic BP decreases by 5–15 mm Hg due to peripheral vascular resistance
Third trimester: gradually returns to prepregnancy values Use of an automated cuff may improve accuracy of measurement, as some pregnant women do not have a fifth Korotkoff sound.
Systolic and diastolic BP may be 16 mm Hg higher when taken while the woman is sitting.
BP readings may decrease in the maternal left lateral position.
Abbreviation: BP, blood pressure.
Data from Jarvis, C. (2016). Physical examination and health assessment (7th ed.). St. Louis, MO: Saunders Elsevier; Ouziunian, J., & Elkayam, U. (2012). Physiologic changes during normal pregnancy and delivery. Cardiology Clinics, 30, 317–329; Tan, E., & Tan, E. (2013). Alterations in physiology and anatomy during pregnancy. Best Practice & Research Clinical Obstetrics & Gynaecology, 27, 791–802.
During pregnancy, blood volume increases by 30% to 50%, or 1,100 to 1,600 mL (Ouziunian & Elkayam, 2012), and peaks at 30 to 34 weeks’ gestation. The increase in blood volume improves blood flow to the vital organs and protects against excessive blood loss during birth. Fetal growth during pregnancy and newborn weight are correlated with the degree of blood volume expansion.
Of the blood volume expansion occurring during pregnancy, 75% is considered to be plasma (King et al., 2015). There is also a slight increase in red blood cell volume
(RBC). The blood volume changes result in hemodilution, which leads to a state of physiologic anemia during pregnancy. As the RBC volume increases, iron demands also increase. Leukocytosis occurs in pregnancy, with white blood cell counts increasing to as much as 14,000 to 17,000 cells per mm3 of blood (Table 29-3). Clotting factors increase as well, creating a risk for clotting events during pregnancy.
Systemic vascular resistance is reduced due to the effects of progesterone, prostaglandins, estrogen, and prolactin. This lowered systemic vascular resistance, in combination with inferior vena cava compression, is partly responsible for the dependent edema that occurs in pregnancy. Epulis of pregnancy, or hypertrophy of the gums accompanied by bleeding, may also occur and is due to decreased vascular resistance and increase in the growth of capillaries during pregnancy (Jarvis, 2016).
Indications and contraindications for prescribing combined estrogen vs. progesterone-only birth control
Progestin-only contraceptives are used continuously; there is no hormone-free interval, as occurs with combined methods. These contraceptive methods have minimal effects on coagulation factors, blood pressure, or lipid levels and are generally considered safer for women who have contraindications to estrogen, such as cardiovascular risk factors, migraine with aura, or a history of VTE. In spite of this belief, the product labeling for some progestin-only products mimics the labeling for products containing estrogen.
The U.S. Medical Eligibility Criteria for Contraceptive Use (CDC, 2010;
see Appendix 11-A) can be used to identify appropriate candidates for progestin- only contraception.
Progestin-only contraceptives do not provide the same cycle control as methods containing estrogen, and unscheduled bleeding is common with all progestin-only methods. Typically, unscheduled bleeding occurs most frequently during the first 6 months of method use, with a substantial number of users becoming amenorrheic by 12 months of use (Hubacher, Lopez, Steiner, & Dorflinger, 2009). Overall blood loss decreases over time, making progestin-only methods protective against iron- deficiency anemia. With appropriate counseling, many women see amenorrhea as a benefit of these methods.
All progestin-only methods are likely to improve menstrual symptoms, including dysmenorrhea, menorrhagia, premenstrual syndrome, and anemia (Burke, 2011). The thickening of cervical mucus seen with progestin methods is protective against PID. Progestin-only contraceptives include the progestin-only pill (POP), an injection, an implant, and three progestin-containing intrauterine devices. The implant and devices are covered in the section on long-acting reversible contraception.
The U.S. Medical Eligibility Criteria for Contraceptive Use (CDC, 2010) is a comprehensive, evidence-based guide for determining whether women have relative or absolute contraindications to contraceptive methods. The Medical Eligibility
Criteria uses the following four classification categories of whether a person can use or should not use a method:
• Category 1: a condition for which there is no restriction for the use of the contraceptive method
• Category 2: a condition where the advantages of using the method generally outweigh the theoretical or proven risks
• Category 3: a condition where the theoretical or proven risks usually outweigh the advantages of using the method
• Category 4: a condition that represents an unacceptable health risk if the contraceptive method is used
Menstrual cycle physiology
The initiation of menstruation, called menarche, usually happens between the ages of 12 and 15. Menstrual cycles typically continue to age 45 to 55, when menopause occurs. Many women find themselves reluctant to discuss the existence and normality of menstruation. The word menstruation has been replaced by a variety of euphemisms, such as the curse, my period, my monthly, my friend, the red flag, or on the rag.
Most women experience deviations from the average menstrual cycle during their reproductive years. As a result, it is not uncommon for women to display certain preoccupations regarding their menstrual bleeding, not only in relation to the regularity of its occurrence, but also in regard to the characteristics of the flow, such as volume, duration, and associated signs and symptoms. Unfortunately, society has encouraged the notion that a woman’s normalcy is based on her ability to bear children. This misperception has understandably forced women to worry over the most miniscule changes in their menstrual cycles. Indeed, changes in menstruation are one of the most frequent reasons why women visit their clinician.
Numerous patterns in the secretion of estrogens and progesterone are possible; in fact, it is difficult to find two cycles that are exactly the same. Studies that include women of different ethnicities, occupations, genetics, nutritional status, and age have demonstrated that the length and duration of the menstrual cycle vary widely (Assadi, 2013; Johnson et al., 2013; Karapanou & Papadimitriou, 2010).
Menarche is the most readily evident external event that indicates the end of one developmental stage and the beginning of a new one. It is now believed that body composition is critically important in determining the onset of puberty and menstruation in young women (Ferin & Lobo, 2012). The ratio of total body weight to lean body weight is probably the most relevant factor, and individuals who are moderately obese (i.e., 20–30% above their ideal body weight) tend to have an earlier onset of menarche (Johnson et al., 2013). Widely accepted standards for distinguishing what are regular versus irregular menses, or normal versus abnormal menses, are generally based on what is considered average and not necessarily typical for every woman.
According to these standards, the normal menstrual cycle is 21 to 35 days with a menstrual flow lasting 4 to 6 days, although a flow for as few as 2 days or as many as 8 days is still considered normal (Ferin & Lobo, 2012).
The amount of menstrual flow varies, with the average being 50 mL; nevertheless, this volume may be as little as 20 mL or as much as 80 mL. Generally, women are not aware that anovulatory cycles and abnormal uterine bleeding (changes in bleeding
outside of normal; see Chapter 24) are common after menarche and just prior to menopause (Ferin & Lobo, 2012; Fritz & Speroff, 2011). Menstrual cycles that occur during the first 1 to 1.5 years after menarche are frequently irregular due to the immaturity of the hypothalamic–pituitary–ovarian axis (Fritz & Speroff, 2011).
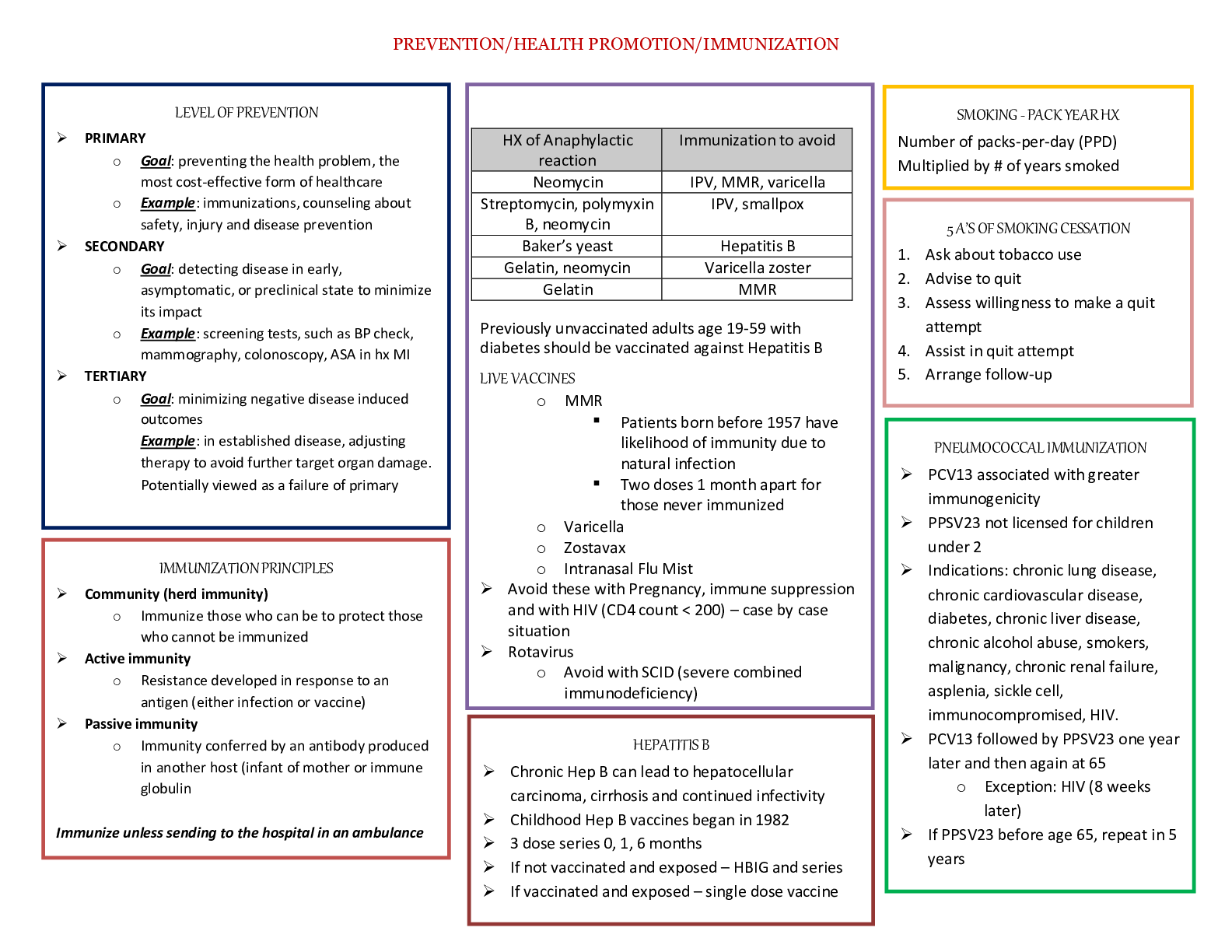
Vaccines during pregnancy
• Live vaccines are contraindicated during pregnancy (MMR, Oral Polio, Varicella & FluMist)
• Injectable influenza vaccine is an inactivated virus and is safe to use in pregnancy
Ask if the woman has ever known anyone with tuberculosis or traveled to areas where tuberculosis is common. If she is at risk, she should receive a tuberculin skin test when she can return in 48 to 72 hours. Past history of varicella is important, as well as the woman’s vaccine history, to determine if she is at risk for chickenpox.
Women can receive vaccines in pregnancy (Table 30-1). The Centers for Disease Control and Prevention (CDC) updates the adult vaccine schedule often, and this information can be easily accessed on its website. The CDC website also includes detailed information about safety of vaccines for travel of local disease outbreaks during pregnancy (CDC, 2014). All women who are pregnant should be offered the influenza vaccine during flu season, though live attenuated influenza vaccine (LAIV [FluMist]) should not be given to pregnant women. All women should be encouraged to receive a tetanus, diphtheria, and acellular pertussis (Tdap) vaccination in the third trimester (CDC, 2016). Other vaccines, such as hepatitis B, can be administered if the woman is at risk (CDC, 2016).
During pregnancy, women have a decreased immune response to pathogens, making them more susceptible to infection. If a woman has cats, she should be careful to avoid contracting toxoplasmosis—an infection that is spread through cat feces. Someone else should change the cat litter box daily to prevent contact with the Toxoplasma
gondii parasite. Wearing gloves while gardening, and careful hand washing are also essential. More information and patient handouts are available for free at the CDC website.
TABLE 30-1 Vaccines in Pregnancy
Recommended Each Preg nancy Rationale Timing
Influenza (flu)a
Women who are pregnant are at Any
increased risk for flu-related gestation
complications. when the
injection is
available
Tetanus, diphtheria, pertussis (Tdap) After maternal vaccination, antibodies cross the placenta and decrease the risk of pertussis infection in the newborn. Third trimester (ideally 27–
36 weeks’ gestation)
Advised If at Risk Rationale Timing
Hepatitis B If the woman is at risk for acquiring HBV, she should be vaccinated. Indications include risk of occupational exposure to blood, treatment for a sexually transmitted infection, more than 1 sex partner in the past 6 months, recent intravenous drug use, and HBsAg– positive sex partner. 3 injections beginning at any point in gestation
Contraindicated Rationale
Measles, mumps, rubella This live virus vaccine has a (theoretical) risk to the fetus.
Varicella This live virus vaccine has a (theoretical) risk to the fetus.
Abbreviations: HBsAg, HBV surface antigen; HBV, hepatitis B virus.
a Live attenuated influenza vaccine (LAIV [FluMist]) should not be given to pregnant women.
Emergency contraception
Sperm can live for up to 5 days in the female reproductive tract, and pregnancy can occur with intercourse 5 days prior to ovulation. The highest risk of pregnancy is in the 48 hours immediately preceding ovulation (Wilcox, Dunson, & Baird, 2000).
However, due to the uncertainty of ovulation timing, emergency contraception is offered if unprotected intercourse (UPI) occurs at any time in the menstrual cycle.
The Yuzpe, levonorgestrel, and ulipristal acetate emergency contraceptive pill (ECP) regimens as well as the copper IUD may all be used within 120 hours of UPI. The Yuzpe and levonorgestrel methods have a dramatic decline in their effectiveness with time and should be used as soon as possible after an event of UPI.
The Yuzpe regimen consists of combined ECPs that
The Yuzpe regimen consists of combined ECPs that must contain at least 100 mcg of ethinyl estradiol and 0.50 mg of levonorgestrel, repeated in 12 hours. A dedicated combined ECP product is not available in the United States, but numerous COCs can be used as combined ECPs (see Table 11-1, footnote i). COCs containing norgestrel
are preferable to those with norethindrone, as failure rates are slightly higher with norethindrone (Zieman et al., 2015). Because the high dose of ethinyl estradiol causes unpleasant side effects, this regimen has largely fallen out of favor.
Until recently, the most widely used emergency contraception method was levonorgestrel ECPs, which contain either a 1.5-mg single dose (Plan B One-Step) or two doses of 0.75 mg taken 12 hours apart (Next Choice and Plan B). Women can take both doses in the two-dose products (Next Choice and Plan B) as a single dose.
Levonorgestrel ECPs are available over the counter to women and men age 17 and older; women 16 and younger need a prescription to obtain them. Levonorgestrel ECPs are more effective than the Yuzpe regimen and have fewer side effects.
Ulipristal acetate (ella), a selective progesterone receptor modulator provided as a single 30-mg dose, is the most effective oral emergency contraception method. The effectiveness of this medication does not decline within the 120-hour window after UPI, as is the case for levonorgestrel and combined ECPs (Fine et al., 2010). Ulipristal acetate is available only by prescription.
The copper IUD can be inserted as long as 5 days after unprotected intercourse. Some contraceptive guidelines recommend its use up to 7 days after UPI (Dunn et al., 2013). This method is rarely utilized as emergency contraception in the United States; however, recent evidence suggests some women might choose the copper IUD if it is offered as an option (Turok et al., 2011). It has the advantage of being highly effective in obese women and providing ongoing contraception.
Efficacy and Effectiveness
Factors influencing the risk of pregnancy when ulipristal acetate or levonorgestrel is used
for emergency contraception include body mass index (BMI), the day of the cycle, and further intercourse during the same menstrual cycle after use of emergency contraception (Glasier et al., 2011).
Women with a BMI greater than 30 have a 2- to 40-fold higher risk of pregnancy after ECP use. Levonorgestrel may be completely ineffective at reducing pregnancy risk in obese women. The efficacy of levonorgestrel and ulipristal acetate further vary according to the stage of the cycle.
Levonorgestrel and ulipristal acetate inhibit ovulation in 96% and 100% of cycles, respectively, when used prior to the onset of the LH surge (Brache, Cochon, Deniaud, & Croxatto, 2013). However, if given after the onset of the LH surge, these medications inhibit ovulation in 14% and 79% of cycles, respectively (Glasier, 2013). Levonorgestrel is no more effective than placebo when used in the critical 5 days preceding ovulation. The risk of pregnancy with ulipristal acetate use is half that seen with use of levonorgestrel (Glasier, 2014).
Both levonorgestrel and ulipristal acetate delay ovulation. If women have repeated acts of UPI after using ECPs, they are at a 4-fold increased risk of pregnancy compared with women who do not have further intercourse within the same cycle.
The copper IUD is by far the most effective of emergency contraception methods, with a pregnancy rate of approximately 1 in 1,000 cases in which it is used for this purpose (Cheng, Che, & Gulmezoglu, 2012).
Safety and Side Effects
Levonorgestrel ECPs, combined ECPs, and ulipristal acetate should not be given to women with a known or suspected pregnancy; there are no other contraindications to their use. The long history of use of levonorgestrel indicates little risk exists if it is inadvertently taken in early pregnancy. There is less experience with ulipristal acetate, although no reasons for concern were raised in the clinical trials. The usual contraindications and precautions for ongoing COC and POP use do not apply to ECPs (CDC, 2010), but the usual contraindications and precautions to copper IUD use do apply when using this method for emergency contraception
(see Appendix 11-A). Neither the copper IUD nor oral emergency contraception methods are considered abortifacients (American College of Obstetricians and Gynecologists, 2014b).
Combined ECPs frequently cause nausea and vomiting, which can be reduced by giving an antiemetic, such as promethazine, prior to treatment. Spotting, changes in next menses, headache, breast tenderness, and mood changes can also occur. These same side effects are sometimes noted with levonorgestrel ECPs but are much less frequent and less severe with this option (Zieman et al., 2015). Headache, dysmenorrhea, nausea, and abdominal pain are the most frequently observed side effects with ulipristal acetate (Fine et al.,
2010; Glasier et al., 2010). The copper IUD can cause the side effects discussed in the section on intrauterine contraception.
Advantages and Disadvantages
Emergency contraception is the only contraceptive method that can be used after intercourse. It cannot be used as an ongoing method of contraception, however, and it provides no STI protection. Access to emergency contraception remains limited because only one method— levonorgestrel ECPs—is available without prescription—and even then it is available only to women 17 and older. Clinicians can increase access to emergency contraception by providing advance prescriptions to all women of reproductive age for ulipristal acetate. Studies have shown that having ECPs at home increases the likelihood that they will be used when needed and does not promote sexual risk taking (Glasier & Baird, 1998; Raine, Harper, Leon, & Darney, 2000). Providing emergency contraception prescriptions over the phone as needed is another way to increase access.
Tier 1, 2 & 3 methods of contraception and efficacy
Tier 1
Intrauterine Devices (IUD)
• Paragard® and T380A® are copper bearing IUDs (Cu-IUD) and are effective up to 10 years
• Mirena®, Skyla® and Liletta® are IUDs that contain levonorgestrel. They are also referred to as LNG-IUSs (levonorgestrel-releasing intrauterine system); slightly more effective than Cu-IUDs; Mirena® is effective for up to 5 years, Skyla® and Liletta® for
up to 3 years; increases cervical mucus to make sperm penetration more difficult
• Works because the device is recognized as a foreign body and a sterile inflammatory response is produced. The inflammatory response has spermicidal
effects decreasing the likelihood of pregnancy (for both LNG-IUSs and Cu-IUD); LNG- IUSs also release progestin making them slightly more effective
• Benefits: highly effective, removes the factor of user consistency and error, long acting and reversible, reduces menstrual flow
• Contraindications: Active pelvic inflammatory disease (PID) or within the last year, suspected or confirmed pregnancy, STD, uterine or cervical abnormality, undiagnosed vaginal bleeding, cancer or history of ectopic pregnancy
• Risks: Endometrial and pelvic infections (first few months after insertion), uterine perforation, heavy or prolonged menstrual periods
• Disadvantages: Requires provider training for insertion, high initial cost
• Side Effects: bleeding and dysmenorrhea are most common, increased bleeding noted more with Cu-IUD; amenorrhea often develops
• Educate woman to check for IUD strings to ensure the device is still in place; expulsion rate is fairly low but occurs most within the first 3 months after insertion
Depot Medroxyprogesterone Acetate (DMPA)
• Depo-Provera® (progestin only); given via injection; lasts 3 months
• Prevent LH surge which inhibits ovulation, thickens cervical mucus and causes the endometrium to atrophy which reduces the likelihood of implantation; minimal
effects on coagulation, blood pressure and lipid levels
• Benefits: Highly effective; reduces menstrual flow and within a year most women have amenorrhea
• Contraindications: Suspected or confirmed pregnancy, known or suspected malignancy of the breast, significant liver disease undiagnosed vaginal bleeding,
history of anorexia, active thrombophlebitis, or current or past history of thromboembolic disorders or cerebrovascular disease
• Risks: Loss of bone mineral density (black box warning: avoid use for more than 2 years), delayed return of fertility
• Side Effects: Headache, depression, breakthrough bleeding and weight gain
• Educate woman calcium with vitamin D and weight bearing exercise to prevent bone mineral loss
• Benefits: Safe for breastfeeding women, considered safer for women who have contraindications to estrogens
• MUST check for pregnancy before starting; Disadvantages: Requires pregnancy testing if the patient does not return every 12 weeks
• Give ONLY within the 1st 5 days of a normal menstrual period-less likely to ovulate during these times
Progestin Implants
Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
• Nexplanon® is a single rod etonogestrel subcutaneous implant, an active metabolite of desogestrel which is effective up to 3 years
• Norplant II® has 2 rods and is effective up to 5 years
• Slow release of progestin to suppress ovulation by inhibiting LH surge
• Benefits: highly effective, removes the factor of user consistency and error, long- acting and reversible, reduces menstrual flow and decreases dysmenorrhea symptoms
• Contraindications: Suspected or confirmed pregnancy, undiagnosed vaginal bleeding, known or suspected malignancy of the breast, significant liver disease, active thrombophlebitis, or current or past history of thromboembolic disorders or
cerebrovascular disease
• Risks: Procedural associated risks are very low; insertion site bruising possible
• Disadvantages: Requires provider training for insertion, high initial cost
• Side Effects: Similar to other progestin-only methods (breakthrough bleeding, amenorrhea, breast tenderness, weight gain)
Sterilization (Tubal ligation or Vasectomy)
• Tubal ligation: Surgical procedure to block the fallopian tubes; various methods of mechanical occlusion which are generally effective immediately; Essure® is the only
transcervical method that can be performed in an office setting by hysteroscopy; Essure ® requires a hysterosalpingogram (HSG) to be done 3 months after the procedure to confirm tubal occlusion; decreased risk of ovarian cancer
• Vasectomy: Surgical procedure to occlude the vas deferens; various methods of occlusion; vasectomy is less invasive than female sterilization; not immediately
effective - the man must wait 3 months before relying on sterilization; usually advised to perform a sperm count before stopping other contraceptive methods
Tier 2 methods essentially include hormonal contraception other than LNG-IUSs and implanted devices. These include:
• Combined oral contraceptive (COC) pills- estrogen and progesterone
• Oral contraceptive pill- progestin only "Minipill"
• Emergency contraception
• Transdermal patch
• Ring
Oral Contraceptive Pills
Since you have already covered the oral contraceptive pills in pharmacology you should be well versed in the mechanism of action, contraindications, risks, benefits and adverse reactions associated with their use. Additionally, your book provides a good deal of information that is useful as a review so we will not spend much time discussing these methods. However, we will circle back to briefly discuss some important take- away information that will be beneficial to you as a provider in determining how to adjust the medication based on the patient's symptoms. Before we do that, let's talk about the patches and rings.
Transdermal
Combined Contraceptive Patch
• Ortho Evra® and Xulane®
• Provides a slow release estrogen & progesterone via the skin; new patch is applied each week for 3 weeks with a patch free time during week 4 (withdrawal period
expected during hormone free time)
• Benefits: Effective, does not require daily dosing, decreased risk of PID and ectopic pregnancies, decreased risk of ovarian and endometrial cancer, improves dysfunctional uterine bleeding (DUB) and dysmenorrhea and improves acne
• Start on 1st day of period or 1st Sunday after period; requires back-up method for 1 week
• Disadvantages: Must remember to change patch the same day every week and remove for 1 week, less effective in women over 200 lbs.
• Contraindications: Same as COC pills
• Risks: Same as COC pills
• Side effects: Same as COC pills
Cervical Ring
• NuvaRing®
• Plastic cervical ring that is placed inside the vagina for 3 weeks which provides a slow release of estrogen and progesterone; removed for 1 week at which time a withdrawal bleed should occur
• Benefits: Effective, does not require daily administration, decreased risk of PID and ectopic pregnancies, decreased risk of ovarian and endometrial cancer, improves dysfunctional uterine bleeding (DUB) and dysmenorrhea and improves acne
• Disadvantages: Woman must be educated on how to apply and remove ring (should fit snugly around the cervix), may be noticeable by patient or partner during
intercourse, not good for woman who is not comfortable with insertion and removal; can be removed prior to intercourse but should be replaced within 3 hours
• Contraindications: Same as COC pills
• Risks: Same as COC pills
• Side effects: Same as COC pills
Tier 3 methods include all of your barrier methods, natural family planning and coitus interruptus. These methods are the least effective in terms of preventing pregnancy with variable rates between them which are outlined in your textbook. The biggest advantage that the barrier methods offer in addition to preventing pregnancy is that they offer protection in preventing STDs.
Etiology, diagnosis, and treatment of amenorrhea
Amenorrhea simply means absence of menses and is part of the spectrum of ovulatory disorders classified as AUB-O. The most common causes of amenorrhea are pregnancy, hypothalamic amenorrhea, and PCOS (American College of Obstetricians and Gynecologists, 2014). According to Fritz and Speroff (2011, p. 436), women meeting any of the following criteria should be evaluated for amenorrhea:
No menses by age 14 in the absence of growth or development of secondary sexual characteristics
No menses by age 16 regardless of the presence of normal growth and development of secondary sexual characteristics
In women who have menstruated previously, no menses for an interval of time equivalent to a total of at least three previous cycles, or 6 months
Amenorrhea typically is categorized as either primary or secondary. Primary amenorrhea is the failure to begin menses by the age of 16. A number of disorders can be treated as soon as they are diagnosed, however, so any girl who has not reached menarche by age 15 years or who has not had a menses within 3 years of thelarche should be evaluated (American College of Obstetricians and Gynecologists, 2014).
Secondary amenorrhea is defined as 3 months without a menses once menses has been established. The American Society for Reproductive Medicine recommends that any woman experiencing 3 months of amenorrhea once the menses is established should be evaluated (American College of Obstetricians and
Gynecologists, 2014). Because primary and secondary amenorrhea can have the same causes, the initial investigation for both is similar.
Physiologic causes of amenorrhea include anatomic defects, ovarian failure, chronic anovulation, anterior pituitary disorders, and central nervous system disorders. Age is an important criterion in making the differential diagnosis of primary versus secondary amenorrhea, and is relevant in determining the types of questions to ask when taking the medical history. Primary amenorrhea in a young woman may be indicative of HPOA disorder or anatomic factors, such as outflow tract obstruction. With primary amenorrhea, the physical examination should focus on identifying the maturation of secondary sex characteristics (e.g., Tanner staging for breast development and pubic hair pattern; see Chapter 2) and establishing outflow tract patency. The question “Have you had any bleeding from the vagina?” can assist in determining primary, secondary, and potential causes. Other important interview questions to consider relate to lifestyle patterns (e.g., exercise, medication, and drug use) and eating habits (e.g., possible eating disorders). A family history of anatomic or genetic abnormalities should be explored as well.
Normal menstrual function requires that four anatomic and structural components are in working order: uterus, ovary, pituitary, and hypothalamus (Fritz & Speroff, 2011). The clinician can then categorize the amenorrhea according to the site or level of disturbance (Fritz & Speroff, p. 438):
Disorders of the genital outflow tract
Disorders of the ovary
Disorders of the anterior pituitary
Disorders of the hypothalamus or central nervous system
The differential diagnosis for women who are not pregnant and who present with amenorrhea is either primary amenorrhea or secondary amenorrhea, although Fritz and Speroff (2011) warn that premature categorization of amenorrhea can lead to diagnostic omissions and, in many cases, unnecessary and expensive diagnostic testing.
Athletic women, particularly long-distance runners, gymnasts, and professional ballet dancers, are at risk for amenorrhea, as are women who have anorexia and other eating disorders (Fritz & Speroff, 2011;
Polotsky, 2010). Typically, amenorrhea occurs as the menstrual cycle stops after the start of an intensive training regimen, although some reports indicate that when intensive training begins prior to menarche, menarche can be delayed by as much as 3 years (Fritz & Speroff, 2011). It is important to understand that it is not exercise in general that causes the amenorrhea, but rather the specific type of exercise (Fritz & Speroff, 2011). For example, swimming is less likely to cause amenorrhea than long-distance running.
Women with a low BMI and low percentage of body fat combined with a high level of intensive physical activity have the highest risk for amenorrhea (Fritz & Speroff, 2011).
The pathophysiology of exercise-induced amenorrhea is complex and most likely due to the combination of low body fat and diminished secretion of GnRH. Lower GnRH levels result in fewer luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which in turn decreases the amount of estrogen produced by the ovaries. The critical weight theory hypothesizes that some critical weight and amount of body fat exist that must be maintained for women to experience regular menstrual cycles (Fritz & Speroff, 2011).
Treat= All women with anovulation require management of this condition: If left untreated, endometrial cancer can occur, regardless of the woman’s age. Typically treatment consists of inducing menses using a progestogen such as medroxyprogesterone acetate 5 to 10 mg daily for the first 12 to 14 days of the cycle. It is important for the woman to know she is not protected against pregnancy during this treatment. If she does not have her menses, she should have a pregnancy test if she has engaged in intercourse during the treatment period. Oral contraceptive pills can also be used to induce a menstrual cycle.
Etiology, diagnosis, and treatment of dysmenorrhea (primary vs. secondary)
One of the most common gynecologic complaints that you will encounter in primary care is dysmenorrhea. Dysmenorrhea refers to painful uterine contractions which occur during menstruation and is one of the most common causes of chronic pelvic pain in women of reproductive ages. It is characterized as cyclical (occurring before or during menses) pain that results from an excessive amount of endometrial prostaglandin production. Prostaglandins stimulate the uterine myometrium which induce uterine irritability and contractions thereby resulting in pain and cramping. Other common symptoms may accompany dysmenorrhea and are associated with premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD), which we will discuss more in a separate section.
Many women experience mild discomfort during menstruation, but the term dysmenorrhea is reserved for women whose pain prevents or interferes with normal activity and requires medication (OTC or prescription). In some women, the pain of dysmenorrhea can be significant and disruptive to school, work and social activities. It is also underreported and undertreated due, largely in part, to negative social and gender stigmas associated with menstruation. In some third world countries, women are still banished from their homes and forced to live in “menstrual huts” during their periods and are without basic hygiene needs. You may even be aware of some of the global efforts to promote health education in order to ameliorate the myths associated with menstruation as well as measures to improve access to sanitary hygiene products.
Primary dysmenorrhea, which is more common than secondary dysmenorrhea, often begins 6 to 12 months after menarche. Typically symptoms are experienced with the onset of bleeding and continue for 8 to 72 hours into the menstrual cycle. Increased endometrial prostaglandin production is believed to be the cause of the associated pain (Lentz, 2007). It is associated with multiple symptoms, including abdominal cramps, headache, backache, general body aches, continuous abdominal pain, and other somatic discomforts. The difference between
primary dysmenorrhea and normal somatic and psychological changes prior to menses is that primary dysmenorrhea is perceived as more severe, with chronic, sometimes debilitating symptoms. There is no evidence of organic pathology in the uterus, fallopian tubes, or ovaries with primary dysmenorrhea. Women usually report repeated symptomology with each cycle. When charting their cycles, it is evident that that pain, bleeding, and disruption of lifestyle occur at regular times in the cycle.
Dysmenorrhea can be classified into 2 categories:
1. Primary-Cyclical menstrual pain with no identifiable pelvic disease
2. Secondary-Cyclical menstrual pain that results from pelvic pathology
Primary dysmenorrhea is almost always associated with ovulatory cycles so it usually does not occur immediately at menarche, but rather within the first 6 months.
Typically the age of onset is between 16-25 years and becomes more severe with age.
Associated primary dysmenorrhea is a diagnosis of EXCLUSION, in other words, secondary causes must be ruled out before rendering the diagnosis.
Primary dysmenorrhea is likely based on history as well as an unremarkable physical exam and a negative pregnancy test.
The most common MISDIAGNOSIS of primary dysmenorrhea is secondary dysmenorrhea due to endometriosis.
Dysmenorrhea that is caused by pelvic pathology is referred to as secondary dysmenorrhea.
Diagnosis of secondary dysmenorrhea includes pelvic pathology such as adenomyosis, leiomyomata, irritable bowel syndrome, interstitial cystitis, and endometriosis (Hoffman, 2008). Almost any process that can affect the pelvic viscera and cause acute or intermittent recurring pain might be a source of cyclic premenstrual pain, including urinary tract infection, pelvic inflammatory disease, hernia, and pelvic relaxation or prolapse
Secondary dysmenorrhea is pain due to an underlying pelvic pathology which may include, but is not limited to: pelvic inflammatory disease, endometriosis, uterine fibroids and adenomyosis.
However, almost any pathology that causes irritation to the pelvic viscera may be a source of pain including bladder and bowel disorders.
Endometriosis is the MOST common cause of secondary dysmenorrhea.
Endometriosis, the most common cause of secondary dysmenorrhea, is a growth or multiple growths (polyps) of endometrial tissue that are found outside of the uterine cavity.
Because the lesions are uterine tissue, they respond to the cyclic hormones of the menstrual cycle which result in bleeding and pain.
The lesions can also attach to adjacent organs such as the ovaries, bowel, bladder or peritoneum.
Uterine fibroids are benign tumors of the uterine myometrium, the smooth muscle of the uterus. Fibroids can range in size from microscopic to very large. Single or multiple fibroids are possible.
Like endometriosis, fibroids can be associated with menorrhagia, infertility and bowel and bladder complaints. Uterine fibroids are the most common indication for hysterectomy in the United States.
Risk factors include heredity, obesity, african american ancestry and a primiparous status (giving birth to only 1 child).
Differentiate between PMS & PMDD.
Symptoms PMS PMDD
Physical Abdominal bloating and Physical: same as PMS but may be more severe
symptoms pain Symptoms can begin immediately after ovulation
Mild weight gain from water Abdominal bloating and pain
retention Headache
Constipation followed by Pelvic pain and cramping
diarrhea at the onset of the Fatigue
menses Extremity edema
Headache Nausea/food cravings
Pelvic pain and cramping
Fatigue
Extremity edema
Nausea/food cravings
Psychologic Depression Marked affective lability
symptoms Anxiety Marked irritability or anger or increased
Anger/irritability interpersonal conflicts
Insomnia Markedly depressed mood, feelings of
Changes in libido hopelessness, or self-deprecating thoughts
Symptoms
PMS
PMDD
Confusion, decrease in mental sharpness Social withdrawal Feelings of low self- esteem/poor self-image
Marked anxiety, tension, feelings of being “keyed up” or “on edge”
Decreased interest in usual activities Subjective sense of difficulty concentrating Lethargy
Insomnia or hypersomnia
A subjective sense of being overwhelmed or out of control
Diagnostic criteria
Symptoms begin up to 7 days prior to menses Remission of symptoms occurs from cycle days 4– 13
Symptoms are significant enough to impair activities of daily living
Symptoms are charted in at least 2 cycles
Symptoms are not due to another disorder
Symptoms are associated with clinically significant distress or interference with work, school, social activities, or relationships with others
The disturbance is not an exacerbation of the symptoms of another disorder (e.g., major depressive disorder)
Criteria should be confirmed by prospective daily ratings during at least 2 symptomatic cycles (the diagnosis may be made provisionally prior to this confirmation)
Symptoms are not due to the direct physiological effects of a substance (e.g., drug abuse, medications other than treatment) or a general medical condition
Abbreviations: PMDD, premenstrual dysphoric disorder; PMS, premenstrual syndrome.
PMS can be defined as a cluster of mild to moderate physical and psychological symptoms that occur during the late luteal phase of menses and resolve with menstruation (Baller et al., 2013; Biggs & DeMuth, 2011). PMDD encompasses cognitive, behavioral, emotional, and negative symptomatic changes that severely impair daily functioning, relationships, parenting, and ability to work in the late luteal menstrual phase
PMS) describes the cyclical recurrence of symptoms that impair a woman’s health, relationships, and occupational functioning. Premenstrual dysphoric disorder (PMDD) is a diagnostic label that applies to a much smaller number of menstruating women experiencing severe PMS with predominantly negative affective symptoms.
Premenstrual Syndrome (PMS) and Premenstrual Dysphoric Disorder (PMDD) are conditions which have both physical and behavioral symptoms that occur during the luteal phase of the menstrual cycle. PMS is common and occurs in the majority of premenopausal women, whereby one or more affective or somatic symptoms occur; PMDD has a far less common incidence. PMDD is considered a more severe form of PMS, where the symptoms significantly disrupt daily functioning. Etiologies are not well understood but evidence that diet, hormones, behavioral and psychosocial factors may all contribute to the development of PMS and PMDD in some way. The treatment of PMS/PMDD depends on the severity of the symptoms. Mild symptoms can be treated with dietary changes (limiting caffeine, alcohol and tobacco or eating
regular small high carbohydrate meals). Exercise, stress management, NSAIDs, vitamin supplements and cognitive behavioral therapies may also be helpful.
Treatment for moderate or severe symptoms include a SSRI (fluoxetine, sertraline, paroxetine, etc.) which can be given continuously or during the luteal phase only, or combined oral contraceptives given continuously or with reduced non-hormone days. GnRH agonist may be also be tried if symptom relief is not achieved with the other medical treatment options. Bilateral oophorectomy and hysterectomy are reserved for refractory cases of moderate to severe PMDD.
Abnormal uterine bleeding terminology
Abnormal Uterine Bleeding (AUB) is a term used to refer to uterine bleeding that is atypical in the frequency, regularity, duration and timing and is a common gynecological disorder. "Normal" menstruation can be classified into the following parameters:
Frequency Every 24-38 days
Regularity +/- 2-20 days
Duration 3-8 days
Quantity 5-80 mL (roughly 3-6 pads per day)
AUB occurs in various “patterns” which are important to first understand before we discuss etiologies.
Structural vs. Nonstructural etiologies of abnormal uterine bleeding
Evaluation and management of abnormal uterine bleeding
History
Obtaining a thorough gynecological history is essential in order to help establish the differential diagnosis for ANY menstrual disorder including AUB. A thorough history should include:
• Age
• Overall health & lifestyle
• Chronic diseases, history of trauma, extreme stressors, exercise regularity (i.e., competitive)
• History of personal or family eating disorders
• Family history of reproductive disorders
• Changes in body weight or in body distribution
• Age at menarche
• Parity
• Menstrual history (regularity, frequency, durations, volume)
• Dysmenorrhea, post-coital bleeding, dyspareunia, infertility, vaginal discharge
• Recent abortion
• Sexual activity
• Contraception (IUD, OCP, hormones)
• Medications
• Symptoms of endocrine disorders (heat intolerance, headaches, weight gain or loss, hair loss, dry skin, increase in shoe or breast size, galactorrhea, etc)
• Symptoms of bleeding disorders (easy bruising, petechiae, etc.)
Physical Examination
• Height, weight, body fat distribution and BMI
• Vital signs (pulse, blood pressure, respiratory rate)
• Color of skin and capillary refill (pallor is suggestive of anemia due to heavy menstrual bleeding)
• Thyroid examination (nodules or goiter)
• Assess for androgen excess (hirsutism, acanthosis nigricans, acne, alopecia)
• Breast exam is warranted if the patient reports breast changes or nipple discharge
• A visual field examination should also be performed in any patient with headaches or galactorrhea (suggestive of pituitary adenoma)
Pelvic Examination
A pelvic exam should be performed on any woman who is or has been sexually active and has irregular or heavy bleeding.
• Visual inspection of the external genitalia (may reveal signs of androgen excess, trauma, or vaginal atrophy due to lack of estrogen)
• A speculum examination should be performed to assess the vagina for foreign bodies, evidence of infection or trauma, as well as to assess the cervix for signs of abnormal growths, lesions or infection
• A bimanual examination should be performed to assess the size and position of the uterus, presence of palpable masses, uterine tenderness and adnexal masses or
tenderness
Diagnostic Studies
Testing decisions should be based on the differential diagnosis that is derived from the patient's history and physical. There are MANY tests and procedures that are available but not all should be performed. Generally speaking, perform the least invasive tests to narrow your differential and start with the most likely causes first. Table 24-4 in your
textbook provides an excellent guide to ordering tests according to the condition that you are trying to rule out. Laboratory tests should be ordered selectively and must always include the patient in the testing and management plan. Tests that may be considered include:
• Pregnancy test (qualitative vs. quantitative; asses for pregnancy or threatened abortion)
• CBC with differential (assess for anemia or clotting disorder)
• PT, aPTT (assess for bleeding disorder)
• Serum ferritin (assess for iron deficiency; only necessary IF anemia is present)
• TSH with reflex FT4 (assess for thyroid dysfunction)
• Prolactin (assess for prolactinoma/pituitary adenoma)
• FSH (assess for menopause or premature ovarian failure)
• Cervical cytology (assess for atypical cells suggestive of dysplasia or cancer; follow current cervical screening guidelines)
• Microscopic exam of vaginal secretion (assess for vaginal candidiasis or bacterial vaginosis)
• STI testing of vaginal secretions (gonorrhea, chlamydia, trichomoniasis)
• Ultrasonography (assess for abnormal masses)
• Endometrial sampling* (assess for endometrial hyperplasia or cancer)
• Hysteroscopy or Laparoscopy* (for biopsy and histology of an identified mass)
(*) Requires a referral to OB/GYN
Provider Tips
• Unopposed estrogen stimulation of the endometrium results in endometrial hyperplasia which increases a woman's risk of endometrial cancer. The most common symptom is post-menopausal bleeding. Therefore, providers should assume that ALL women with post-menopausal bleeding OR women ≥ 45 years with AUB have endometrial cancer UNTIL proven otherwise with endometrial biopsy
• In women of reproductive age, a complication of pregnancy must always be
considered as a cause for abnormal uterine bleeding. Therefore, all women with AUB should be considered pregnant UNTIL proven otherwise
Management and Treatment
Definitive management of AUB should focus on normalizing bleeding, correcting anemia (if present), preventing cancer and restoring quality of life for the patient. The treatment options that are available for AUB are based on the cause and may include pharmacologic measures such as contraceptives, GnRHs, NSAIDs and antifibrinolytics. Non-pharmacological interventions typically include surgical methods such as endometrial ablation, thermal endometrium destruction, uterine artery embolization and hysterectomy. Referral is indicated for an unidentified cause of AUB, complicated cases, refractory treatment, and surgery.
Breast mass types and diagnostic studies
The most common benign breast masses are fibroadenomas and cysts. Lipomas, fat necroses, phyllodes tumors, hamartomas, and galactoceles may also be encountered.
Fibroadenomas, which are composed of dense epithelial and fibroblastic tissue, are usually nontender, encapsulated, round, movable, and firm. They are the most common type of breast mass in adolescents and young women. Their incidence decreases with increasing age, but they still account for 12% of
masses in menopausal women (Pearlman & Griffin, 2010). Multiple fibroadenomas occur in 10% to 15% of cases (Vashi, Hooley, Butler, Geisel, & Philpotts, 2013). A proposed etiology for formation of these masses is the effect of estrogen on susceptible tissue (Vashi et al., 2013).
Cysts are fluid-filled masses that are most commonly found in women aged 35 to 50 years (Berg, Sechtin, Marques, & Zhang, 2010). They are thought to result from cystic lobular involution. Although many of these lesions can be dismissed as benign simple cysts requiring intervention only for symptomatic relief, complex cystic and solid masses require biopsy.
A lipoma is an area of fatty tissue that may occur in the breast or other areas of the body, including the arms, legs, and abdomen. Lipomas typically occur in the later reproductive years (Grobmeyer, Copeland, Simpson, & Page, 2009). Fat necrosis is usually the result of trauma to the breast, whether as a result of external force against the tissue (e.g., a seat belt in a motor vehicle accident) or subsequent to surgical manipulation of tissue (Grobmeyer et al., 2009)
Phyllodes tumors form from periductal stromal cells of the breast and present as a firm, palpable mass. These typically large and fast-growing masses account for fewer than 1% of all breast neoplasms.
Phyllodes tumors, which can range from a benign mass to a sarcoma, are usually seen in women aged 30 to 50 years (Pearlman & Griffin, 2010). Hamartomas are composed of glandular tissue, fat, and fibrous connective tissue; the average age at presentation for these masses is 45 years (Sevim et al., 2014).
Galactoceles are milk-filled cysts that usually occur during or after lactation. They result from duct dilation and often have an inflammatory component (Vashi et al., 2013). See Color Plate 12 for an example of a breast mass.
Type
of Mass Typical Physical Examination Findings Tissue Sampling Findings
Fibroadenoma Discrete, smooth, round or oval, nontender, mobile Ductal epithelium, dense stroma, numerous elongated nuclei without fat
Cyst Discrete, tender, mobile; size may fluctuate with the menstrual cycle Cyst fluid and inflammation
Lipoma Discrete, soft, nontender; may or may not be mobile Fatty tissue
Fat necrosis Ill defined, firm, nontender, nonmobile Necrotic fat with inflammation
Type
of Mass Typical Physical Examination Findings Tissue Sampling Findings
Phyllodes tumor Discrete, firm, round, mobile, findings similar to a fibroadenoma, but mass is usually larger; may observe stretching of skin due to rapid tumor growth Stromal hypercellularity with glandular and ductal elements
Hamartoma Discrete, nontender, nonmobile; may be nonpalpable with incidental diagnosis on imaging studies Glandular tissue, fat, and fibrous connective tissue
Galactocele Discrete, firm, sometimes tender Fat globules
If the physical examination suggests a palpable area of concern, order an ultrasound if the woman is younger than age 30, and a diagnostic mammogram with or without an ultrasound if she is 30 years or older. If the mass is suspicious for malignancy on physical examination and the woman is least 30 years of age, order a mammogram and ultrasound (NCCN, 2015a; Salzman et al., 2012). Ultrasound helps to distinguish a cystic mass from a solid mass, but is not as accurate as tissue sampling. Mammography can be used to detect nonpalpable abnormalities if a woman is of appropriate screening age or has a solid mass. Palpable breast masses may not be visible with diagnostic imaging tests, however, so these tests cannot rule out malignancy.
Biopsy is required to definitively ascertain whether a mass is solid versus cystic, and benign versus malignant. A fine-needle aspiration (FNA) biopsy is a minimally invasive way to differentiate solid and cystic masses, and provides for cytologic evaluation of a palpable mass. FNA biopsy may also be therapeutic if the mass is filled with fluid.
Tissue sample findings for benign breast masses are described in Table 15-2. If cytologic evaluation does not yield definitive findings, a more invasive method of tissue sampling (Table 15-3) is required to rule out breast cancer or determine the type of tumor if the mass is benign
Differential diagnosis for pelvic pain
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
Diagnoses of Gynecologic Origin Nongynecologic Diagnoses
Endometriosis
Chronic pelvic inflammatory disease (PID) Dysmenorrhea, primary and secondary Pelvic adhesions
Pelvic congestion Mittelschmerz Vulvodynia Uterine prolapse Ovarian cyst
Ovarian remnant syndrome Adenomyosis
Fibroids Ovarian cancer Cervical cancer
Torsion of adnexa Tubo-ovarian abscess Uterine fibroids Ectopic pregnancy
Abortion, threatened or incomplete
Gastrointestinal
Irritable bowel syndrome (IBS) Diverticulitis
Constipation Bowel obstruction Appendicitis Colon cancer Gastroenteritis
Genitourinary
• Interstitial cystitis
Urinary tract infection Urinary retention Renal calculi Pyelonephritis Ureteral lithiasis Bladder neoplasm
Musculoskeletal
• Scoliosis
Radiculopathy Arthritis Herniated disk Hernia
• Abdominal wall hematoma
Other
• Aortic aneurysm
Pelvic thrombophlebitis
• Acute porphyria
• Abdominal angina
Psychiatric, depression Somatization disorder
Prior or current physical or sexual abuse
The most common gynecologic-related causes of chronic pelvic pain identified by laparoscopy are endometriosis (see Chapter 26) and adhesions (Rapkin & Nathan, 2012). Other common causes include ovarian remnant, retained ovary syndrome, pelvic congestion syndrome, pelvic relaxation causing prolapse of gynecologic organs (e.g., uterine prolapse; see Chapter 25), subacute salpingo- oophoritis (see Chapter 20), cancer of gynecologic origin (see Chapter 27), and ovarian hyperstimulation syndrome (OHSS).
Pelvic adhesions are coarse bands of tissue that connect organs to other organs or to the abdominal wall in places where there should be no connection. Adhesions can be caused by previous surgeries, infection, or endometriosis (Hoffman, 2012; Rapkin & Nathan, 2012; Styer, 2013). They may be the etiology for infertility, dyspareunia, and bowel obstruction (Styer, 2013). Currently, the causal role of adhesions in pelvic pain is unknown. The myriad of symptoms range from mild intermittent abdominopelvic pain to constant pain with gastrointestinal (constipation, bloating, dyschezia), gynecologic (dyspareunia, dysmenorrhea, focal lateral or
central pelvic and adnexal pain), and musculoskeletal symptoms (abdominal wall tenderness) (Styer, 2013). If symptoms are exacerbated during specific portions of the menstrual cycle (e.g., menses, luteal phase, follicular phase), then medical therapy should be considered with use of hormonal suppression of the cycle (Styer, 2013). If symptoms are constant, then use of nonsteroidal anti-inflammatory drugs (NSAIDs) and other forms of analgesics should be considered, with possible referral to
a pain specialist (Styer, 2013).
Etiology, diagnosis, and treatment of common breast disorders
Clinical signs and symptoms of ectopic pregnancy
Ectopic pregnancy is a potentially life-threatening form of pregnancy complication resulting from implantation of the fertilized egg outside the uterus, usually in the fallopian tube. With a prevalence of approximately 2% of reported pregnancies (Marion & Meeks,
2012), ectopic pregnancy is a leading differential diagnosis when a woman has lower abdominal pain in the first trimester. Risk factors include a history of pelvic inflammatory disease or infertility. However, if a woman who is newly pregnant is experiencing lower quadrant pain, it is important to rule out ectopic pregnancy even if she does not have risk factors. Any women with cervical motion tenderness on bimanual examination should be evaluated
for ectopic pregnancy, as should any woman early in pregnancy with pelvic or abdominal pain. Bleeding, ranging from spotting to the amount that occurs during a menstrual period, can also be a symptom (Crochet et al., 2013). If the woman’s hCG level is more than 3,000 mIU/mL, a gestational sac should be visible within the uterus. Ectopic pregnancies tend to have slowly rising hCG levels that increase but do not double within 48 hours.
Ectopic pregnancy is the implantation of a fertilized ovum in locations other than the uterine cavity. It is the second leading cause of maternal mortality in the United States (Marion & Meeks, 2012). Approximately 95% of all ectopic pregnancies occur in the fallopian tube (American College of Obstetricians and Gynecologists,
2008, reaffirmed 2014). Growth of the fetus in the fallopian tube puts the woman who is pregnant at high risk for pregnancy loss, tubal rupture, excessive blood loss, and future infertility due to tubal scarring. Box 31-1 summarizes risk factors associated with ectopic pregnancy.
Pelvic and abdominal pain and unexplained vaginal bleeding are the primary symptoms experienced by most women with ectopic pregnancy. The pain may be described as vague, sharp, diffuse, or unilateral. The woman may have had a time of amenorrhea, and pregnancy may or may not already be diagnosed. A ruptured ectopic pregnancy is characterized by a sudden onset of vaginal bleeding and sharp, severe, unilateral abdominal pain. Following rupture, symptoms of significant blood loss and resulting shock may include hypotension, shoulder pain, and breast tenderness.
Physical findings associated with ectopic pregnancy include cervical motion tenderness, a uterus that is not enlarged, adnexal mass, and adnexal tenderness. Diagnosis may take several steps and should be managed by a maternal–fetal specialist. Immediate data to collect to aid in the diagnosis include a transvaginal ultrasound to determine the contents of the uterus, pregnancy test, complete blood count, and β-hCG levels.
Differential diagnoses include appendicitis, pelvic inflammatory disease, bowel irritability or obstruction, cholecystitis, pyelonephritis, and ovarian torsion.
Breast cancer risk factors
BOX 15-1 Risk Factors for Breast Cancer
• Female
• Advancing age
• Personal history of invasive breast cancer, ductal carcinoma in situ, or lobular carcinoma in situ
• Family history of invasive breast cancer, ductal carcinoma in situ, or lobular carcinoma in situ, especially in first-degree relatives
• Inherited detrimental genetic mutations
• Biopsy-confirmed proliferative breast lesions with atypia
• Dense breast tissue on mammogram
• High-dose radiation to chest, especially during puberty or young adulthood
• Menarche before age 12 years
• Menopause at age 55 years or older
• Nulliparity
• First full-term pregnancy after age 30 years
• Current use of combined oral contraceptives (likely due to detection bias of regular screening)
• Use of combined estrogen–progestogen hormone therapy after menopause
• Weight gain leading to overweight or obese status after age 18 years
• Physical inactivity
• Consumption of one or more alcoholic beverages per day
• Jewish ancestry (Ashkenazi)
Breast cancer screening guidelines (USPSTF)
American Academy of Family Physicians (AAFP) and US Preventative Services Task Force (USPSTF)
• Decision to start screening mammography in women between 40-49 years should be an individual one. Women who place a higher value on the potential benefits than on the potential harms can choose to begin screening every 2 years
• Women 50-74 years should undergo screening mammography every two years
• Women > 75 years screening mammography is not recommended as current evidence is insufficient to assess the balance of benefits and harms
Diagnosis and treatment of non-STI vaginitis (vulvovaginal candidiasis, bacterial vaginosis)
VC- In addition to obtaining a thorough history of the woman’s symptoms (their onset and course), it is valuable to identify predisposing risk factors. The physical examination includes a thorough inspection of the vulva and vagina. A speculum examination and microscopic examination of vaginal secretions with saline and KOH are performed. The characteristic pseudohyphae may be seen on a wet smear done with normal saline, although these organisms may sometimes be confused with other cells and artifacts.
Pseudohyphae are best seen on the KOH wet smear (Figure 19-2). Vaginal pH can be tested and is normal with VVC (3.5–4.5). If the pH is greater than 4.5, the clinician should suspect coinfection with trichomoniasis or BV. In recurrent or resistant cases, vaginal cultures for Candida can confirm the diagnosis, identify less common species, and redirect treatment when candidiasis is suspected but the KOH prep is negative (CDC, 2015).
Given the wide availability of OTC therapies, many symptomatic women presume their symptoms are VVC related and choose to self-treat rather than see a healthcare provider. When self-treatment is ineffective and women seek professional advice, accurate identification of the offending organism(s) is sometimes obscured by intravaginal OTC preparations.
Candida albicans is a normal inhabitant of the vagina and the most common cause of VVC (see Color Plate 16). The most common symptom of VVC is vulvar and possibly vaginal
pruritus (Table 19-1). This itching may be mild or intense, interfere with rest and activities, and occur during or after intercourse. Some women report a feeling of dryness. Others may experience painful urination as the urine flows over the vulva; this symptom usually occurs in women who have excoriation resulting from scratching. Most often the discharge is thick, white, and lumpy with the consistency of cottage cheese. Often the discharge is found in patches on the vaginal walls, cervix, and labia. The vulva is commonly red and swollen, as are the labial folds, vagina, and cervix. Although no characteristic odor is associated with VVC, sometimes a yeasty or musty smell occurs. A number of antifungal preparations are available for the treatment of VVC (Table 19-2), and no single brand is significantly more effective than
another. Vaginal creams and suppositories recommended for treatment of this condition are oil based, so they may weaken latex condoms and diaphragms. Many effective topical azole drugs are available as OTC products; they have cure rates ranging from 80% to 90% (Sobel, 2007). Only women who have been previously diagnosed with VVC and who are experiencing the same symptoms should attempt self-treatment with OTC medications. Any woman whose symptoms persist or who develops a recurrence of symptoms within 2 months of treatment should be evaluated by a clinician (CDC, 2015). Unnecessary or inappropriate use of OTC preparations is common and can lead to delays in treating other causes of vulvovaginitis.
BOX 19-1 Criteria for Clinical Diagnosis of Bacterial Vaginosis
Clinical diagnosis of bacterial vaginosis is based on the presence of three out of four of the following Amsel criteria:
• White, thin adherent vaginal discharge
• pH ≥ 4.5
• Positive whiff/KOH test
• Clue cells on microscopic examination (more than 20% of epithelial cells are clue cells)
Vaginitis Recommended Regimens Alternative Regimens
BV Metronidazole 500 mg orally twice a day for 7 days OR
Metronidazole gel 0.75%, one full applicator (5 g) intravaginally, daily for 5 days OR
Clindamycin cream 2%, one full applicator (5 g) intravaginally, at bedtime for 7 days Pregnant Women
Metronidazole 500 mg orally twice a day for 7 days OR
Metronidazole 250 mg orally three times a day for 7 days OR
Clindamycin 300 mg orally twice a day for 7 days Tinidazole 2 g orally once daily for 2 days OR
Tinidazole 1 g orally once daily for 5 days OR
Clindamycin 300 mg orally twice a day for 7 days OR
Clindamycin ovules 100 g intravaginally once at bedtime for 3 days
Pregnant Women
None
Recurrent BV Retreat with original therapy Metronidazole 0.75% intravaginally once weekly for 4–6 months OR Metronidazole 2 g orally and fluconazole 150 mg orally in a single dose once monthly
Uncomplicated VVC Over-the-Counter Intravaginal Agents Clotrimazole 1% cream 5 g intravaginally for 7–14 days OR
Clotrimazole 2% cream 5 g intravaginally
for 3 days OR
Miconazole 2% cream 5 g intravaginally for 7 days OR
Miconazole 4% cream 5 g intravaginally for 3 days OR
Miconazole 100 mg vaginal suppository, one suppository daily for 7 days OR Miconazole 200 mg vaginal suppository, one suppository daily for 3 days OR Miconazole 1,200 mg vaginal suppository, one suppository for 1 day OR Tioconazole 6.5% ointment 5 g intravaginally in a single application Prescription Intravaginal Agents Butoconazole 2% cream (single-dose
bioadhesive product), 5 g intravaginally for a single application OR
Terconazole 0.4% cream 5 g intravaginally for 7 days OR
Terconazole 0.8% cream 5 g intravaginally for 3 days OR
Terconazole 80 mg vaginal suppository, one suppository for 3 days
Oral Agent
Fluconazole 150 mg oral tablet, one tablet in single dose
Pregnant Womena
Topical azole therapy, applied for 7 days
Complicated VVC Recurrent VVC Initial Therapy
Longer duration of initial therapy, such as topical azole for 7–14 days OR Fluconazole 150 mg orally every third day for a total of 3 doses (days 1, 4, and 7) OR
Itraconazole 200 mg orally twice daily for 3 days
Recurrent VVC Maintenance Therapy Fluconazole 150 mg orally weekly for 6 months OR
Itraconazole 100–200 mg daily for 6 months OR
Miconazole 1,200 mg vaginal suppository, one suppository weekly for 6 months OR Intermittent use of topical treatments Severe VVC
Topical azole for 7–14 days OR Fluconazole 150 mg in 2 sequential doses, second dose 72 hours after initial dose
Non-albicans VVC Initial Therapy
Optimal treatment unknown;
options include nonfluconazole azole drug (oral or topical) for 7–14 days
Recurrent Non-albicans VVC Boric acid 600 mg in gelatin capsule vaginally once daily for 14 days
HIV Infection
Should not differ from that of seronegative women
the gold standard for BV diagnosis remains the Gram stain. Although cytology reports may include comments such as “predominance of coccobacilli consistent with shift in vaginal flora,” Pap testing is both nonspecific and nonsensitive for diagnosing BV. Furthermore, vaginal cultures are not useful because BV is multibacterial in origin and cultures are nonspecific Alternative Therapies for Bacterial Vaginosis, Vulvovaginal Candidiasis, or Vulvovaginal Symptoms
Intervention Dosage Administration Use
Gentian violet Few drops in water, 0.25–2% Douche or local application VVC
Vinegar (white) 1 teaspoon per quart of water
1–2 tablespoons per quart of water Douche every 5–7 days or twice a day for 2 days Douche 1–2 times/week VVC or BV
Acidophilus culture 2 tablespoons per pint of water Douche twice a day VVC
Vitamin C 500 mg 2–4 times daily Orally VVC
Acidophilus tablet 40 million–1 billion units (1 tab) daily Orally VVC
Yogurt 1 application to labia or in vagina Hourly as needed VVC
Goldenseal 1 teaspoon in 3 cups warm water, strain and cool Douche BV (not safe for use in pregnancy)
Garlic clove 1 peeled clove wrapped in cloth dipped in olive oil Overnight in vagina, change daily BV
Intervention Dosage Administration Use
Boric acid powder 600 mg in gelatin capsule Every day in vagina for 14 days (toxic if ingested orally) BV
Sassafras bark Steep in warm water Compress Wash affected area VVC
Cold milk, cottage cheese, yogurt Compress or insert in vagina Apply to affected area Pruritus
Calendula Steep in boiling water than cool Douche Vulvovaginal inflammation
Tea tree oil Soaked tampon or 1 suppository Douche or 1 suppository daily Vulvovaginal inflammation
Clindamycin cream is preferred in case of allergy or intolerance to metronidazole or tinidazole; however, women should be advised that clindamycin may weaken latex condoms or contraceptive diaphragms. Treatment of sexual partners of women who have BV is not recommended because it does not affect the woman’s response to treatment or the likelihood of relapse or recurrence. The CDC recommends that all women with BV be tested for HIV and other sexually transmitted infections
Etiology, incidence, transmission, clinical findings, diagnosis, associated risks and treatment of STIs
Genital herpes is one of the most common STIs in the United States, but its exact prevalence is unknown because HSV is not a reportable infection. It is the primary cause of genital ulcer disease in the United States. According to CDC surveillance data, the overall prevalence rate of genital HSV in non-Hispanic Caucasian women is approximately 15%. Non-Hispanic women of African descent have the highest reported
rates among all females—approximately 50%, more than 3 than the rates
in (CDC, 2014b). HSV prevalence also increases with a higher
number of lifetime sex partners: Seroprevalence has been reported as 5.4% in women with one lifetime sex partner, 18.8% in women with two to four lifetime partners, 21.8% in women with five to nine lifetime partners, and 37.1% in women with 10 or more lifetime partners.
Although HSV rates are high, more than 85% of individuals who are positive for HSV-2 antibodies report that they have never had a clinician tell them they had genital herpes (CDC, 2014b). In fact, most people with HSV-2 antibodies have never been diagnosed with genital herpes. Despite their mild or unrecognized infections, they intermittently shed the HSV-2 virus in the genital tract. As a result, most genital herpes infections are transmitted by individuals who do not know they have HSV-2 or who do not have symptoms at the time of transmission (CDC, 2015d). An initial or primary genital herpes infection characteristically has both systemic and local symptoms and lasts approximately 3 weeks. Flu-like symptoms with fever, malaise, and myalgia first appear
about a week after exposure, peak within 4 days, and subside over the next week. Multiple genital lesions develop at the site of infection, which is usually the vulva, but may be present anywhere in the anogenital area. Other commonly affected sites are the perianal area, vagina, and cervix. The lesions begin as small painful blisters or vesicles that become “unroofed,” leaving behind ulcerated lesions (see Color Plate 20).
Individuals with a primary herpes infection often develop bilateral, tender, inguinal lymphadenopathy; vulvar edema; vaginal discharge; and severe dysuria (Hawkins et al., 2016).
Ulcerative lesions last 4 to 15 days before crusting over, and new lesions may develop over a period of 10 days during the course of the infection. Cervicitis is also common with initial HSV-2 infections. The cervix may appear normal, or it may be friable, reddened, ulcerated, or necrotic if cervical lesions are present. A heavy, watery to purulent vaginal discharge is possible. Extragenital lesions may be present because of autoinoculation. Urinary retention and dysuria may occur secondary to autonomic involvement of the sacral nerve root (Hawkins et al., 2016).
Women experiencing recurrent episodes of genital herpes typically develop only local symptoms that are less severe than those associated with the initial infection due to the initial immune response. Systemic symptoms are usually absent with recurrences, although the characteristic prodromal genital tingling is common. Recurrent lesions are unilateral, are less extensive than the original lesions, and usually last 7 to 10 days without prolonged viral shedding. Lesions begin as vesicles and progress rapidly to ulcers (Hawkins et al., 2016). Very few women with recurrent infection have cervicitis.
Establishing a diagnosis of genital HSV can be challenging. Many individuals with HSV do not have overt symptoms. Thus, in making the diagnosis of genital herpes, a history of exposure to a person with HSV infection is important, although infection from an asymptomatic individual is common. A history of viral symptoms, such as malaise, headache, fever, or myalgia, is suggestive of HSV infection. Likewise, local symptoms such as vulvar pain, dysuria, itching, or burning at the site of infection, and painful genital lesions that heal spontaneously are very suggestive of HSV infection. The clinician should also ask about prior history of a primary infection, prodromal symptoms, vaginal discharge, dysuria, and dyspareunia.
During the physical examination, the clinician should assess for inguinal and generalized lymphadenopathy and elevated temperature. Carefully inspect the entire vulvar, perineal, vaginal, and cervical areas for vesicles, ulcers, or crusted areas. A speculum examination may be very difficult for the patient because of the extreme tenderness often associated with genital herpes. Any genital lesion that is extremely tender should be tested for HSV even if the appearance is not consistent with the classic herpes lesions.
Although a diagnosis of HSV infection may be suspected from the woman’s history and physical examination, it can be confirmed only by laboratory studies. Isolation of HSV in cell culture or by polymerase chain reaction (PCR) is the preferred test in women who have genital ulcers or other mucocutaneous lesions. Viral culture is less sensitive than PCR, with the best culture yield being found during a primary infection or if the
specimen is taken during the vesicular stage of the infection—the sensitivity of a culture declines rapidly as lesions begin to heal. Both culture and PCR can be negative in a person with HSV infection because the virus is shed only intermittently (CDC, 2015d). Type-specific serologic tests are useful in confirming a clinical diagnosis given the frequency of false-negative HSV cultures, especially in women with healing lesions or recurrent infection. Antibodies are present within the first several weeks after infection and persist indefinitely. Clinicians should be certain to specifically request serologic
type-specific glycoprotein G (IgG)–based assays. Serologic test options include laboratory-based assays and point-of-care tests using capillary blood or serum during a clinic visit. The sensitivity of these tests varies from 80% to 98%, and false-negative results can occur, especially in early-stage infection when antibodies are still developing. If there is a strong clinical suspicion of HSV in the presence of a negative result, testing can be repeated within a few months. The specificity of these assays is 96% or greater, and false-positive results can occur in individuals with a low likelihood of HSV infection.
Serologic screening for HSV is not recommended for the general population, but should be considered in women who experience recurrent or atypical genital symptoms with negative HSV cultures, have a clinical diagnosis of genital herpes without laboratory confirmation, present for STI evaluation (especially if they have multiple sexual partners), or have HIV. Testing should also be considered for asymptomatic partners of women with HSV infection (CDC, 2015d). All women with genital herpes should be tested for other STIs, including chlamydia, gonorrhea, syphilis, and HIV. These drugs do not cure the infection, however, nor do they alter the subsequent risk, frequency, or rate of recurrence after discontinuation. Three antiviral medications provide clinical benefits for genital herpes: acyclovir, valacyclovir, and famciclovir (Table 20-4). Topical antiviral therapy is not recommended due to its minimal benefits (CDC, 2015d).
TABLE 20-4 Treatment of Genital Herpes
Primary Infectiona Recurrent Infection Suppressive Therapy
Acyclovir 400 mg Acyclovir 400 mg orally 3 Acyclovir 400 mg orally 2 times a day
orally 3 times a day times a day for 5 days or
for 7–10 days or Famciclovir 250 mg orally 2 times a day
or Acyclovir 800 mg orally 2 or
Acyclovir 200 mg times a day for 5 days Valacyclovir 500 mg orally once a day (may be
orally 5 times a day or less effective than other valacyclovir or
for 7–10 days Acyclovir 800 mg orally 3 acyclovir dosing regiments in patients who
or times a day for 2 days have 10 or more episodes per year)
Famciclovir 250 mg or or
orally 3 times a day Famciclovir 125 mg orally Valacyclovir 1 gm orally once a day
for 7–10 days 2 times a day for 5 days
or or
Valacyclovir 1 gm Famciclovir 1,000 mg
orally 2 times a day orally 2 times a day for 1
for 7–10 days day
or
Famciclovir 500 mg orally
Primary Infectiona
Recurrent Infection Suppressive Therapy
once, followed by 250 mg 2 times a day for 2 days or
Valacyclovir 500 mg orally 2 times a day for 3 days or
Valacyclovir 1 gm orally once a day for 5 days
Chancroid is a bacterial infection of the genitourinary tract caused by the gram- negative bacteria Haemophilus ducreyi. Chancroid is uncommon in the United States, with only 10 cases being reported in the country in 2013. This number may be an underestimate, however, reflecting the fact that the causative organism of chancroid is difficult to culture (CDC, 2014b, 2015d). Chancroid is a genital ulcer and, therefore, is a risk factor for HIV transmission. The major way chancroid is acquired is through sexual contact and trauma (CDC, 2015d), although infection through autoinoculation of fingers or other sites occasionally occurs. The incubation period for the infection, though not well established, usually ranges from 4 to 7 days but may be as long as 3 weeks (CDC, 2015d).
Most women with chancroid present with a history of a painful macule on the external genitalia that rapidly changes to a pustule and then to an ulcerated lesion (see Color Plate 21). They may also develop enlarged unilateral or bilateral inguinal nodes known as buboes. After 1 to 2 weeks, the skin overlying the lymph node becomes erythematous, the center necroses, and the node becomes ulcerated
A probable diagnosis of chancroid can be made when one or more painful genital ulcers are present; there is no evidence of syphilis (per dark-field examination of ulcer exudate or serologic testing at least 7 days after ulcer onset); the clinical presentation, ulcer appearance, and regional lymphadenopathy (if present) are typical for chancroid; and HSV testing of the exudate is negative (CDC, 2015d). Because chancroid is more prevalent in certain geographic areas and less common in the United States, women should be asked about recent travel to or sexual activity with a partner from Africa or the Caribbean, where chancroid outbreaks are more common (Hawkins et al., 2016).
Definitive diagnosis of chancroid is difficult because the organism can be identified only by culture on a special medium that is not used routinely; even when this technique is used, the test’s sensitivity is less than 80% (CDC, 2015d). Testing for HIV and syphilis should be performed at the time of diagnosis and repeated in 3 months if initial testing was negative. The recommended treatments for chancroid are azithromycin 1 gm orally in a single dose, ceftriaxone 250 mg IM in a single dose, ciprofloxacin 500 mg orally twice a day for 3 days, or erythromycin base 500 mg orally 4 times a day for 7 days (CDC, 2015d). Women with comorbid HIV infection may require repeated or longer therapy (CDC, 2015d).
Women should be reexamined 3 to 7 days after beginning therapy. If treatment is successful, symptomatic improvement should be apparent within 3 days of starting therapy. Objective clinical improvement should be noticeable on examination 7 days
after treatment, although it may take more than 2 weeks for complete healing of large ulcers.
Pubic lice Recommended treatments for pediculosis pubis include permethrin 1% cream rinse and pyrethrins with piperonyl butoxide. These medications are applied to the affected areas and washed off after 10 minutes. If symptoms do not resolve within a week and treatment failure is thought to be due to drug resistance, an alternative regimen consists of Malathion 0.5% lotion applied for 8 to 12 hours and washed off. Oral ivermectin (250 mcg/kg) taken initially and repeated in 2 weeks is another alternative regimen, although this medication has limited ovicidal activity. Taking ivermectin with food increases this medication’s bioavailabilit
CHLAMYDIA
Chlamydia, which is caused by the bacterium Chlamydia trachomatis, is the most commonly reported nationally notifiable infection in the United States and the most common bacterial STI. More than 1.45 million cases were reported to the CDC in 2013, with at least that many more estimated to have gone undetected (CDC, 2014b).
Sexually active adolescents and women aged 15 to 24 years of age have nearly three times the prevalence of chlamydia as women aged 25 and 39 years, and women are infected at a rate of two times that of men. The prevalence of chlamydia is six times higher in black women than in white women (CDC, 2014b). Risk factors for this infection include multiple sexual partners and failure to use barrier methods of contraception. The most serious complication of chlamydial infections for women is PID (see the section on PID later in this chapter).
When assessing patients for chlamydia, in addition to obtaining information about any risk factors, inquire about the presence of any symptoms, while recognizing that chlamydia is usually asymptomatic. Women experiencing symptoms may report vaginal spotting or postcoital bleeding, mucoid or purulent cervical discharge, urinary frequency, dysuria, lower abdominal pain, or dyspareunia. Bleeding results from inflammation and erosion of the cervical columnar epithelium. Symptoms of chlamydia infection in women may mimic those of a urinary tract infection (UTI). In sexually active women who present with urinary symptoms only, it may be prudent to test a urine sample for chlamydia if urine is already being collected for dipstick analysis or culture (Hawkins et al., 2016).
Physical examination findings of abdominal guarding, referred pain, or rebound tenderness upon abdominal examination should raise the level of suspicion for PID. Cervical friability may be detected with the speculum examination. Discharge, if present, is characteristically mucopurulent (see Color Plate 24). During the bimanual examination, a woman may report cervical motion tenderness (pain with cervical movement), and the examiner may detect adnexal fullness and uterine tenderness.
These findings are also suggestive of PID (Hawkins et al., 2016).
In recent years, the CDC has expanded recommendations for chlamydia screening among asymptomatic women. All sexually active women younger than 25 years should be screened for chlamydia annually (CDC, 2014b, 2015d). Women 25 years and older with risk factors (e.g., new or multiple partners, partner with an STI, partner with other partners) should also be screened.
Chlamydia testing can be performed using first-catch urine or swab specimens from the endocervix or vagina. Screening procedures for chlamydial infection include NAATs, cell culture, direct immunofluorescence, enzyme immunoassay (EIA), and nucleic acid hybridization tests. NAATs are the preferred technique because they provide the highest sensitivity (see Chapter 6). Although less sensitive than urine or cervical/vaginal swabs, NAATs can also be used with liquid-based Pap tests. All patients with chlamydia should be tested for other STIs, including gonorrhea, syphilis, and HIV (CDC, 2015d). TABLE 20-5 Treatment of Chlamydial Infections
Recommended Regimens Alternative Regimens
Azithromycin 1 gm orally in a single dose
or
Doxycycline 100 mg orally 2 times a day for 7 days Erythromycin base 500 mg orally 4 times a day for 7 days
or
Erythromycin ethylsuccinate 800 mg orally 4 times a day for 7 days
or
Levofloxacin 500 mg orally once daily for 7 days
or
Ofloxacin 300 mg orally 2 times a day for 7 days
Women should be advised to abstain from sex until their sexual partners are treated and to wait 7 days after single-dose treatment or until completion of a 7-day regimen before resuming sexual activity.
Trichomoniasis is caused by Trichomonas vaginalis, an anaerobic one-celled protozoan with characteristic flagellae. This organism most commonly lives in the vagina in women, and in the urethra in men. Among women presenting with vaginitis symptoms, 4% to 35% will have trichomoniasis. The prevalence of T. vaginalis has been reported by researchers as approximately 4.1% for white women and 16.1% for black women (Rogers et al., 2014). Higher rates have been seen in specific populations of women, including those who are incarcerated and women who seek care at STI clinics (Alcaide et al., 2015). Trichomoniasis is sexually transmitted during vaginal– penile intercourse or vulva-to-vulva contact. Nonsexual transmission is possible but rare. Trichomoniasis is strongly associated with an increased risk of HIV transmission (Rogers et al., 2014; Van Der Pol et al., 2008).
Although trichomoniasis is often asymptomatic, women can experience a characteristically yellow to greenish, frothy, mucopurulent, copious, malodorous discharge. Inflammation of the vulva, vagina, or both may be present, and the woman may have irritation, pruritus, dysuria, or dyspareunia. Typically, the discharge worsens during and after menstruation (CDC, 2015d).
In addition to the history of current symptoms, a careful sexual history, including information on last intercourse and last sexual contract, should be obtained from the woman with suspected trichomoniasis. Any history of similar symptoms in the past and treatment used should be noted. The clinician should determine whether the woman’s partners were treated and whether she has engaged in subsequent relations with new partners. Additional important information includes the last menstrual period, method of contraception, condom use, and use of other medications (Hawkins et al., 2016).
Inspect the external genitalia for excoriation, erythema, edema, ulceration, and lesions. On speculum examination, note the quantity, color, consistency, and any odor of the vaginal discharge. In women with trichomoniasis, the cervix and vaginal walls may demonstrate characteristic “strawberry spots,” or tiny petechiae, especially after prolonged infection (see Color Plate 23), and the cervix may bleed on contact. In severe infections, the vaginal walls, the cervix, and occasionally the vulva may be acutely inflamed. The pH of vaginal discharge is elevated (Carcio & Secor,
2015; Hawkins et al., 2016).
Diagnosis is usually made by visualization of the typical one-celled flagellate trichomonads on microscopic examination of vaginal discharge (Figure 20-1), although this method has a sensitivity of only approximately 51% to 65%. The slide must be viewed immediately to ensure optimal results. Nonmotile trichomonads are more challenging to recognize. Microscopic examination of the wet mount may also reveal increased numbers of white blood cells, and a strong amine odor will be produced with the addition of potassium hydroxide (KOH) to the specimen. Point-of-care tests are also available that typically have higher sensitivity (more than 82%) and specificity (more than 95%) (CDC, 2015d).
Culture is a sensitive and highly specific method of diagnosis, but is no longer routinely performed because of the availability of nucleic acid amplification tests (NAATs). Culture should be performed when trichomoniasis is suspected but cannot be confirmed with microscopy or NAAT is not available. Although T. vaginalis may be an incidental finding on a Pap test, this test is not considered diagnostic (even with liquid-based specimens), and confirmatory testing is still needed. All patients with trichomoniasis should be tested for other STIs, including chlamydia, gonorrhea, syphilis, and HIV (CDC, 2015d).
The nitroimidazoles are the only antimicrobial medications that are effective against T. vaginalis. The recommended treatment for trichomoniasis is metronidazole 2 gm orally in a single dose or tinidazole 2 gm orally in a single dose. Tinidazole is equivalent or superior to metronidazole in terms of cure and symptom resolution, and has fewer gastrointestinal side effects, but is more expensive than metronidazole. Topical metronidazole is less effective and not recommended.
Most reoccurrences of trichomoniasis are thought to be due to reinfection. If single-dose metronidazole treatment fails and reinfection is excluded, metronidazole 500 mg orally twice a day for 7 days should be prescribed. If infection persists, consider metronidazole or tinidazole 2 gm orally for 7 days. If infection persists, consultation with a specialist is warranted (CDC, 2015d).
Gonorrhea, which is caused by the aerobic, gram-negative diplococcus Neisseria gonorrhoeae, is the second most commonly reported bacterial STI in the United States, after chlamydia. In 2013, 333,004 cases of gonorrhea were reported in the United States, although it is estimated that more than twice this number occurred but were not diagnosed or reported. The rate of infection was slightly higher in men than in women (109.5/100,000 versus 102.4/100,000, respectively). Gonorrhea rates are highest among adolescents and young adult women aged 15 to 24 years. The rate
of gonorrhea in black women is more than 12 times the rate in white women (CDC, 2014b).
Gonorrhea is almost exclusively transmitted by sexual activity, primarily through genital- to-genital contact; however, it is also spread by oral-to-genital and anal-to-genital contact. Sites of infection in females include the cervix, urethra, oropharynx, Skene’s glands, and Bartholin’s glands. In addition to age, other risk factors for this infection include early onset of sexual activity and multiple sexual partners. The main complication of gonorrheal infections is PID. Women may also develop a pelvic abscess or Bartholin’s abscess. Disseminated gonococcal infection (DGI) is a rare (0.5–3%) complication of
untreated gonorrhea. DGI occurs in two stages: The first stage is characterized by bacteremia with chills, fever, and skin lesions; it is followed by the second stage during which the patient experiences acute septic arthritis with characteristic effusions, most commonly in the wrists, knees, and ankles
BOX 20-6 Treatment of Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
Ceftriaxone 250 mg IM in a single dose
or
Cefixime 400 mg orally in a single dosea
or
Other single-dose injectable cephalosporin regimensb (ceftizoxime 500 mg IM, cefoxitin 2g IM with probenecid 1 gm orally, or cefotaxime 500 mg IM)
plus
Azithromycin 1 gm orally in a single dose (preferred)
or
Doxycycline 100 mg orally 2 times a day for 7 days
Women with gonorrhea often remain asymptomatic, with as many as 80% of women having no symptoms from this infection (Hawkins et al., 2016). When symptoms are present, they are often less specific than the symptoms in men. Women may report dyspareunia, a change in vaginal discharge, unilateral labial pain and swelling, or lower abdominal discomfort. Later in the infection’s course, women may describe a history of purulent, irritating vaginal discharge, or rectal pain and discharge. Menstrual irregularities may be the presenting symptom, with longer, more painful menses being noted. Women may also report chronic or acute lower abdominal pain. Unilateral labial pain and swelling may indicate Bartholin’s gland infection (see Chapter 19), whereas periurethral pain and swelling may indicate inflamed Skene’s glands. Infrequently, dysuria, vague abdominal pain, or low backache prompts women to seek care. Later symptoms may include fever (possibly high), nausea, vomiting, joint pain and swelling, or upper abdominal pain (liver involvement) (Hawkins et al., 2016; Marrazzo & Cates, 2011).
Women may develop a gonococcal rectal infection following anal intercourse, in which case they may report symptoms of profuse purulent anal discharge, rectal pain, and
blood in the stool. Rectal itching, fullness, pressure, and pain are also commonly noted symptoms. Women with gonococcal pharyngitis may appear to have viral pharyngitis, as some individuals will have a red, swollen uvula and pustule vesicles on the soft palate and tonsils similar to streptococcal infections (Hawkins et al., 2016).
Physical examination is individualized based on the woman’s presenting symptoms. The clinician should obtain vital signs and perform a general skin inspection for signs of classic DGI lesions, which are painful necrotic pustules on an erythematous base, approximately 1 mm to 2 cm in diameter. Inspect the pharynx and oral cavity for erythema, edema, and lesions. Assess for cervical lymphadenopathy. Palpate the abdomen for masses, tenderness, and rebound tenderness. During the speculum examination, inspect the vaginal walls for discharge and redness, and examine the cervix for mucopurulent discharge, ectopy, and friability (see Color Plate 25). During the bimanual examination, observe for cervical motion tenderness, uterine tenderness, adnexal tenderness, and adnexal masses—all of these findings are associated with PID (Hawkins et al., 2016).
Annual screening for gonorrhea is recommended for all sexually active women younger than 25 years. Women who are 25 or older should be screened based on risk factors such as inconsistent or absent condom use, new or multiple partners, partner with an STI or other partners, and exchange of sex for drugs or money. Clinicians should also inquire about recent travel that included sexual partners outside the United States (CDC, 2015d).
Gonorrhea testing can be performed by culture and NAATs. NAATs can be performed using urine or swab specimens from the endocervix or vagina. Although the U.S. Food and Drug Administration (FDA) has not formally approved NAATs for use in the rectum or pharynx, some laboratories have established performance specifications for using these tests with specimens from these sites. NAAT products vary, however, and clinicians must be certain that the test they are using is appropriate for the specimen type (CDC, 2015d). Culture is also available for the detection of gonorrhea infection of the rectum and pharynx. All patients with gonorrhea should be offered testing for other STIs, including chlamydia, syphilis, and HIV.
Diagnosis and treatment of vaginal masses
Asymptomatic simple ovarian cysts less than 10 cm in diameter, including functional cysts and benign neoplasms, have a low probability of malignancy and can be followed with serial imaging. There is little evidence-based guidance for timing of repeat ultrasounds, but they are generally obtained at 3-or 6-month intervals to establish stability (Ackerman, Irshad, Lewis, & Munazza, 2013). Many functional cysts will resolve within 3 months. In one study, benign-appearing cysts (e.g., unilocular or cysts with septations but no solid component) resolved within 1 year of follow-up in approximately 40% of participating women (Pavlik et al., 2013). Women who are receiving serial follow-up should be educated to report any increase in pain or other symptoms (e.g., bowel or bladder status). While unlikely to promote resolution of an existing cyst, hormonal contraceptives can be used to control repeated episodes of
symptomatic functional cysts. Women with pelvic pain symptoms that suggest adnexal mass should be asked sufficient history questions to allow the clinician to rule out the most morbid or life-threatening conditions quickly, including ectopic pregnancy, appendicitis, ovarian torsion or infection, and malignancy. Key topics from the general health and gynecologic history include the specific characteristics of the symptoms the
woman is experiencing; her menstrual, sexual, and contraceptive history; any changes in vaginal bleeding or discharge; a pain scale; and a family history of risk factors such as malignancy or endometriosis.
Taking the woman’s vital signs will aid in ruling out infection, fever, and severe pain or shock. Systemic or atypical lymphadenopathy may suggest malignancy or infection. Unexplained weight gain or a disproportionate change in abdominal girth without weight gain may indicate large tumor growth.
Inspect, auscultate, and palpate the abdomen. Large masses may cause visible changes in the abdomen or may be palpable abdominally or on bimanual vaginal and rectal examination. Guarding or rebound tenderness can indicate peritoneal irritation.
Perform a complete pelvic examination, documenting the location, size, shape, texture, mobility, and tenderness of any palpable mass. While pelvic and/or rectal examination are indicated, be aware that their sensitivity is limited, especially in women with higher body mass index (ACOG, 2007, reaffirmed 2013).
Pregnancy testing is essential in women of reproductive age. Gonorrhea and chlamydia testing and complete blood count are warranted if a tubo-ovarian abscess is suspected. While imaging is the first-line assessment for adnexal pathology, blood testing plays a role in multivariate index assays that have been developed in an attempt to predict malignant potential reliably and limit unnecessary intervention (Dodge et al.,
2012; Elder et al., 2014). Tests appropriately ordered in primary care while specialist consultation is pending include cancer antigen 125 (CA-125), alpha fetoprotein (AFP), lactate dehydrogenase (LDH), and human chorionic gonadotropin (hCG) (ACOG, 2007, reaffirmed 2013). The CA-125 test is not reliable as a stand-alone modality for distinguishing benign and malignant masses, especially in premenopausal women, as the level of this protein is also elevated by endometriosis, fibroids, pelvic inflammatory disease, and other benign findings (ACOG, 2007, reaffirmed 2013). In addition, no biomarkers have been demonstrated to be accurate for screening purposes, as they can lead to unacceptably high rates of unnecessary intervention, without significant reduction in mortality.
Transvaginal ultrasound remains the first-line imaging modality for evaluation of ovarian masses (ACOG, 2007, reaffirmed 2013). Ultrasound will classify a mass as cystic, solid, or complex. Three-dimensional ultrasound may improve acuity, while color Doppler ultrasound as an adjunct can assess measurement of blood flow in and around a mass (Dodge et al., 2012). MRI and computed tomography (CT) imaging are typically reserved for specific indications such as determination of the origin of an associated non-ovarian pelvic mass (MRI) or evaluation for potential metastases (CT) (ACOG, 2007, reaffirmed 2013).
Asymptomatic simple ovarian cysts less than 10 cm in diameter, including functional cysts and benign neoplasms, have a low probability of malignancy and can be followed with serial imaging. There is little evidence-based guidance for timing of repeat ultrasounds, but they are generally obtained at 3-or 6-month intervals to establish stability (Ackerman, Irshad, Lewis, & Munazza, 2013). Many functional cysts will resolve within 3 months. In one study, benign- appearing cysts (e.g., unilocular or cysts with septations but no solid component) resolved within 1 year of follow-up in approximately 40% of participating women (Pavlik et al., 2013).
Women who are receiving serial follow-up should be educated to report any increase in pain or other symptoms (e.g., bowel or bladder status). While unlikely to promote resolution of an existing cyst, hormonal contraceptives can be used to control repeated episodes of symptomatic functional cysts.
Women can be informed that complex and solid ovarian masses have been shown to resolve spontaneously in as many as 80% of women (Pavlik et al., 2013). This type of mass does have a higher risk for malignancy, however, and the woman should be referred from primary care to general gynecologic or gynecologic oncology for in-depth assessment
maging studies such as transvaginal ultrasound may be helpful in determining ovarian size and general tumor composition in women with an identified pelvic mass (ACS, 2015c; American College of Obstetricians and Gynecologists, 2011b). Transvaginal ultrasound has value in the determination of advanced ovarian cancer, but its ability to provide accurate detection in the early stage of the disease is poor and as a screening method it does not reduce the number of deaths from ovarian cancer (Moyer & USPSTF, 2012). The finding of a palpable
pelvic mass combined with a positive transvaginal ultrasound and elevated CA-125 level may improve the rate of accurate detection, although research is ongoing to determine how use of imaging methods such as transvaginal ultrasound combined with CA-125 measurements affect survival rates of ovarian cancer for both average-and high-risk populations
Further imaging studies such as CT scan or MRI of the abdomen, pelvic ultrasound, intravenous pyelogram, barium enema, or colonoscopy may be considered to determine metastasis. Tumor biopsy is not recommended, as it may cause tumor cells to further disseminate into the peritoneal cavity For women who are postmenopausal and who have a pelvic mass, the CA-125 measurement may be helpful in predicting a higher likelihood of malignancy, but a normal finding does not preclude lack of disease. Approximately 50% of stage I tumors and 20% of advanced disease are associated with normal CA-125 values
Nationally reportable infections chancroid, chlamydia, gonorrhea, hepatitis, HIV, and syphilis
Pelvic exam cytology (classification and treatment)
• CIN 1 lesions involve the initial 1/3 of the epithelial layer
• CIN 2 lesions involve 1/3 to less than 2/3 of the epithelial layer
• CIN 3 lesions involve 2/3 of the epithelial layer to full thickness
Atypical Squamous Cells of Undetermined Significance (ASCUS)
o Term used when cells do not appear normal, but the cause is unknown
o Does not exclude CIN 1-3 and cancer
o Reflex testing for HPV on abnormal PAP results and repeat testing is based on those
results
Atypical Glandular Cells (AGCs)
o More common in older women (ages 40-69 years)
o 1/3 of cases are associated with pre-malignancy or malignancy
o Risk of cancer increases with age
o Refer for Endometrial Biopsy
Low-Grade Squamous Intraepithelial Lesions (LSIL)
o Cervical cells are mildly abnormal
o Usually caused by a low risk HPV infection
o Appropriateness of repeat screening vs. referral for diagnostic testing is largely
dependent upon whether or not the woman is HPV + and age
High-Grade Squamous Intraepithelial Lesions (HSIL)
o Abnormal cervical cells which are more likely to be associated with premalignancy and malignancy.
o Refer immediately for cervical biopsy and treatment (Colposcopy or LEEP procedure)
Clinical findings, diagnosis, associated risks and treatment of pelvic inflammatory disease
The severity and extent of symptoms that women with PID experience vary widely. Historically, the abrupt onset of acute lower abdominal pain following menses has been considered the characteristic presenting symptom of PID. More recently, it has been recognized that symptoms of this infection can be very mild and nonspecific. Commonly reported symptoms include abdominal, pelvic, and low back pain; abnormal vaginal discharge; intermenstrual or postcoital bleeding; fever; nausea and vomiting; and urinary frequency (Brunham et al., 2015). Women may report levels of pain ranging from minimal discomfort to dull, cramping, and intermittent pain to severe, persistent, and incapacitating pain. Pelvic pain is usually exacerbated by the Valsalva maneuver, intercourse, or movement. Symptoms of STIs in a woman’s partners also should be noted.
Although treatment regimens vary with the infecting organism, broad-spectrum antibiotics are generally administered. Several antimicrobial regimens have proved to be effective, and no single therapeutic regimen appears to be superior to the others (Table 20-6). Substantial clinical improvement should occur within 72 hours of beginning treatment. Women who do not respond within this time frame should be reevaluated to confirm the diagnosis of PID; they may also need hospitalization (if being treated on an outpatient basis), additional testing, and surgical intervention. Women
who do not respond to oral therapy and have a confirmed diagnosis of PID should be treated with an inpatient or outpatient parenteral regimen. Women on parenteral regimens can usually be transitioned to oral therapy 24 to 48 hours after they begin to show clinical improvement.
Minimal pelvic examinations should be done during the acute phase of PID, and analgesics can be given for pain. During the recovery phase, the woman should restrict her activity and make every effort to obtain adequate rest and consume a nutritionally sound diet. Women with PID who had a positive test for gonorrhea or chlamydia should have repeat testing for these pathogens 3 to 6 months after treatment (CDC, 2015d).
BOX 20-7 Diagnosing Pelvic
Inflammatory
Disease
Empiric treatment of PID should be initiated in sexually active young women and other women at risk for STIs if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be found, and if one or more of the following minimum criteria are present on pelvic examination:
• Cervical motion tenderness
• Uterine tenderness
• Adnexal tenderness
One or more of the following additional criteria can be used to enhance the specificity of
the minimum criteria and support a diagnosis of PID:
• Oral temperature > 101°F (38.3°C)
• Abnormal cervical or vaginal mucopurulent discharge
• Presence of abundant numbers of white blood cells on saline microscopy of vaginal fluid
• Elevated erythrocyte sedimentation rate
• Elevated C-reactive protein level
• Laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis
The most specific criteria for diagnosing PID include the following conditions:
• Endometrial biopsy with histopathologic evidence of endometritis
• Transvaginal sonography or magnetic resonance imaging techniques showing thickened, fluid-filled tubes with or without free pelvic fluid or tubo-ovarian complex,
or Doppler studies suggesting pelvic infection (e.g., tubal hyperemia)
• Laparoscopic abnormalities consistent with PID
Parenteral Regimens Oral/Intramuscular Regimens
plus
Doxycycline 100 mg orally or IV every 12 hours
or
Clindamycin 900 mg IV every 8 hours
plus
Gentamicin loading dose IV or IM (2 mg/kg of body weight), followed by a maintenance dose (1.5 mg/kg) every 8 hours. Single-day dosing (3–5 mg/kg) may be substituted.
Alternative parenteral regimen: Ampicillin/sulbactam 3 gm IV every 6 hours plus
Doxycycline 100 mg orally or IV every 12 hours Metronidazole 500 mg orally 2 times a day for 14 days
or
Cefoxitin 2 gm IM in a single dose and probenecid 1 gm orally administered concurrently in a single dose
plus
Doxycycline 100 mg orally 2 times a day for 14 days
with or without
Metronidazole 500 mg orally 2 times a day for 14 days
or
Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime)
plus
Doxycycline 100 mg orally twice a day for 14 days
witha or without
Metronidazole 500 mg orally twice a day for 14 days
Health education is central to effective management of PID. Women should abstain from sexual activity until treatment has been completed, symptoms have resolved, and sexual partners have been adequately treated. Male sexual partners within the past 60 days preceding the onset of symptoms should be evaluated, tested, and treated presumptively for gonorrhea and chlamydia (CDC, 2015d). Clinicians should explain to women the nature of their infection and encourage them to adhere to all therapy and prevention recommendations, emphasizing the necessity of taking all medication, even if their symptoms resolve before the course of therapy is completed. Any potential problems that would prevent a woman from completing a course of treatment, such as lack of money for prescriptions or lack of transportation to return to a clinic for follow-up appointments, should be identified and the importance of follow-up visits emphasized.
The woman diagnosed with PID will need supportive care, because PID is so closely tied to sexuality, body image, and self-concept. Her feelings need to be discussed, and her partners included in the counseling when appropriate (Hawkins et al., 2016).
Special Considerations
Pregnancy
Pregnant women who have PID are at significant risk for maternal morbidity and preterm birth. They should be hospitalized and treated as inpatients with parenteral antibiotics. Doxycycline is known to discolor teeth and should be avoided in the second and third trimesters. Consultation with an infectious disease specialist is warranted when a woman has multiple antibiotic allergies (CDC, 2015d).
Women Using an Intrauterine Device
Many women use either copper-containing or levonorgestrel-releasing IUDs for contraception (see Chapter 11). An increased risk of PID is seen in the first 21 days after IUD insertion. If a woman who is using either type of IUD is also diagnosed with PID, the device does not need to be removed immediately; indeed, it often does not need to be removed at all. Treatment should be initiated with a recommended antibiotic regimen. If no improvement is seen with 48 to 72 hours after beginning treatment, the IUD should be removed
Cervical cancer screening guidelines (USPSTF)
American Academy of Family Physicians (AAFP) and US Preventative Services Task Force (USPSTF)
• Start screening at age 21; women less than 21 years should not be screened regardless of risk factors including sexual activity with liquid based cytology or convention PAP test
• Women ages 21-29 years should have cervical cytology performed every 3 years
• Women ages 30-65 years should have cervical cytology performed every 3 years
• HPV co-testing in women less than 30 years is not recommended; women who want to extend their screening interval, HPV co-testing every 5 years is an option
• Primary high-risk HPV testing, as an alternative to co-testing or cytology alone is recommended every 5 years for women ages 30-65 years
Primary vs. suppression therapy for HSV
Genital herpes is a chronic and recurring condition for which there is no known cure. Systemic antiviral drugs may partially control the symptoms and signs of HSV infections when used for primary or recurrent episodes, and they may completely control symptoms when used as daily suppressive therapy. These drugs do not cure the
infection, however, nor do they alter the subsequent risk, frequency, or rate of recurrence after discontinuation. Three antiviral medications provide clinical benefits for genital herpes: acyclovir, valacyclovir, and famciclovir (Table 20-4). Topical antiviral therapy is not recommended due to its minimal benefits (CDC, 2015d).
Systemic antiviral therapy should be given to all individuals experiencing their first genital herpes episode. Most people with a symptomatic first episode of genital HSV-2 infection will experience recurrent episodes of genital lesions; by comparison, recurrence is less common among individuals with genital HSV-1 infection. Lifelong, intermittent, asymptomatic, genital shedding occurs in those persons who have HSV-2 infection. Recurrent genital herpes can be treated with daily suppressive therapy, which decreases the frequency of recurrences and the risk of transmitting HSV, or episodic therapy may be implemented when lesions occur to help them heal more quickly.
Episodic therapy should be started within one day of when the lesion begins or during the prodromal symptoms if present. Individuals using episodic therapy should be provided with a prescription or medication in advance to facilitate immediate treatment of outbreaks. All women who have a history of HSV and desire suppressive therapy should be offered treatment if they do not have any contraindications to antiviral medications (CDC, 2015d).
Oral analgesics, such as aspirin or ibuprofen, may be used to relieve pain and systemic symptoms associated with initial infections. Any topical agents should be used with caution, because the mucous membranes affected by herpes are very sensitive.
Ointments containing cortisone should be avoided. Women should be informed that occlusive ointments may prolong the course of infections. During asymptomatic periods, condoms and suppressive therapy can be used to reduce the risk of transmission to partners who do not have HSV infection. Researchers have established the effectiveness of
antiviral suppressive therapy among discordant couples (Lebrun-Vignes et al., 2007; Le Cleach et al., 2014). All women who are diagnosed with genital HSV should be informed of the availability of suppressive therapy that can help prevent transmission of the virus to partners.
TABLE 20-4 Treatment of Genital Herpes
Primary Infectiona Recurrent Infection Suppressive Therapy
Acyclovir 400 mg Acyclovir 400 mg orally Acyclovir 400 mg orally 2 times a day
orally 3 times a day 3 times a day for 5 days or
for 7–10 days or Famciclovir 250 mg orally 2 times a day
or Acyclovir 800 mg orally or
Acyclovir 200 mg 2 times a day for 5 days Valacyclovir 500 mg orally once a day (may be
orally 5 times a day or less effective than other valacyclovir or acyclovir
for 7–10 days Acyclovir 800 mg orally dosing regiments in patients who have 10 or more
or 3 times a day for 2 days episodes per year)
Famciclovir 250 mg or or
orally 3 times a day Famciclovir 125 mg Valacyclovir 1 gm orally once a day
for 7–10 days orally 2 times a day for
or 5 days
Valacyclovir 1 gm or
orally 2 times a day Famciclovir 1,000 mg
for 7–10 days orally 2 times a day for
Primary Infectiona
Recurrent Infection Suppressive Therapy
1 day
or
Famciclovir 500 mg orally once, followed by 250 mg 2 times a day
for 2 days
or
Valacyclovir 500 mg orally 2 times a day for 3 days
or
Valacyclovir 1 gm orally once a day for 5 days
Anticipatory Guidance - birth to adolescent The aim of primary care for children is to promote health, growth, and development. One mechanism for addressing safety issues and parental concerns ahead of problems is to institute standard anticipatory guidance. Standard anticipatory guidance should be a routine part of well-childcare, and many resources exist for current anticipatory guidance information, such as the Bright Futures program. The website is noted below. Anticipatory guidance should be age appropriate and deal with common concerns that can be anticipated at upcoming ages. Anticipatory guidance can be organized into areas of injury and violence prevention, nutrition, sleep, and developmental or behavioral issues and categorized by visit date. The American Academy of Pediatrics recommends well-child visits at 2 weeks and then at 2, 4, 6, 9,
12, 15, 18, and 24 months, annually up to age 6, and every 2 years from age 6 through adolescence.
Topics and needs of anticipatory guidance can vary according to family needs. For example, limiting media time and pediatric obesity education may be needed for one family, while another may need more education on discipline. Careful history taking is key. Clinicians must take the time to address all standard areas and additional parent concerns, keeping in mind that handouts often go unread by families. Standard anticipatory guidance forms per visit can be found at the Bright Futures website.
Growth and development –birth to age 17
Weight
Typically, infants gain 150 to 210 g (approximately 5 to 7 ounces) weekly during the first 6 months of life. By 6 months, the infant’s birth weight doubles. During the next 6 months, weight gain slows. By 1 year of age, the infant’s weight triples from birth weight for an average of 9.75 kg or 21.5 pounds.
Height
Infant length increases an average of 2.5 cm (1 inch) a month during the first 6 months but slows during the next 6 months. While weight gain is steady and gradual, length increase occurs in sudden spurts. By 6 months of age, infants average 65 cm (25.5 inches) in length and increase to 72 cm or 29 inches by 12 months of age—almost a 50% increase from birth— due to truncal not leg increases.
Head Circumference
Like the rest of the growing infant, head circumference growth is rapid. During the first 6 months of life, head circumference increases approximately 1.5 cm (0.6 inches) per month. During the second 6 months of age, head circumference slows to only 0.5 cm (0.2 inches) per month. On average, the head circumference is 43 cm (17 inches) at 6 months and increases to 46 cm (18 inches) by 12 months, which represents a 33% increase from birth. During this time, the cranial sutures close. The posterior fontanel closes between 6 and 8 weeks of age while the anterior fontanel closes around 14 months, with a range between 12 and 18 months of age. During the first 12 months of life, the brain size increases by 2.5 times. Developmental milestone achievement illustrates brain growth and differentiation.
Tanner Staging
Pubic Hair Scale (both males and females)
• Stage 1: No hair
• Stage 2: Downy hair
• Stage 3: Scant terminal hair
• Stage 4: Terminal hair that fills the entire triangle overlying the pubic region
• Stage 5: Terminal hair that extends beyond the inguinal crease onto the thigh
Female Breast Development Scale
• Stage 1: No glandular breast tissue palpable
• Stage 2: Breast bud palpable under areola (1st pubertal sign in females)
• Stage 3: Breast tissue palpable outside areola; no areolar development
• Stage 4: Areola elevated above contour of the breast, forming “double scoop” appearance
• Stage 5: Areolar mound recedes back into single breast contour with areolar hyperpigmentation, papillae development and nipple protrusion
Male External Genitalia Scale
• Stage 1: Testicular volume < 4 ml or long axis < 2.5 cm
• Stage 2: 4 ml-8 ml (or 2.5-3.3 cm long), 1st pubertal sign in males
• Stage 3: 9 ml-12 ml (or 3.4-4.0 cm long)
• Stage 4: 15-20 ml (or 4.1-4.5 cm long)
• Stage 5: > 20 ml (or > 4.5 cm long)
Well child visit
Well- Child History ( Comprehensive, Ongoing)
1. • Patient-identifying information/statement 2.
1. • Identify if this is a new or established patient/family
2. • Child age, sex/gender
3. • Accompanying adult(s) 3.• Reason for the visit
4.
1. • Highlight parental (and child) concerns/priorities 5.• Date of last visit
6.
1. • Interval history (with an established patient/family, seek an “update” of the comprehensive history on record)
7. • Past health/medical history 8.
1. • Prenatal/birth/neonatal history
2. • Childhood illness/injury
3. • Hospitalization/surgery/procedures
4. • Allergies (food, medication, environment)
5. • Immunizations
6. • Medications (prescription, OTC, folk/herb, complementary/alternative therapies)
9. • Prior screening/results
10. • Review of systems—begin with global questions in each system; pursue areas of concern in further detail
11. • Current health 12.
1. • General habits/day-to-day functioning—nutrition, sleep, activity, elimination
2. • Development/milestones—affective, cognitive, physical
3. • Preventative health history—screenings, immunizations, health protection activities
13. • Family history 14.
1. • Family structure/function 2.
1. • Parenting
3. • Family health history
4. • Family ethnic/cultural beliefs/practices
5. • Family health habits (e.g., literacy, smoking, seatbelts, helmets, guns)
15. • Household/environment 16.
1. • Family function—identify family members, role strain, or significant family changes.
2. • Safety/risks—injury, exposure to violence, adverse childhood experiences, toxic exposures, social determinants of health, housing, and food security
17. Health supervision (routine well-child) visits are a core component of pediatric primary care because they provide ongoing opportunities to assess the health and function of the child and family. Each visit typically includes a health history, physical exam, screenings, and sharing of anticipatory guidance. Unlike ill-child encounters, during which the aim is to attend to the presenting malady, the health supervision visit is multifaceted, focusing on health promotion and anticipatory guidance, disease prevention, and disease detection. Each pediatric health supervision visit is guided by knowledge of growth patterns, developmental milestones, individual and age-related disease risk factors, and parent/family priorities and needs. Through ongoing
assessment, the health and developmental trajectory for each child can be plotted and compared with normative data, much like length/height and weight, and any variation can be quickly attended to.
18. The timing and focus of health supervision visits are typically aligned with the American Academy of Pediatrics (AAP) Periodicity Schedule (https://www.aap.org/en- us/Documents/periodicity_schedule.pdf), which serves as a general guideline. Embedded in each visit is ongoing disease detection, which involves two techniques: surveillance and screening. Surveillance is the systematic collection, analysis, and interpretation of data for the purpose of prevention, because findings from health surveillance guide primary prevention measures. Surveillance is a continuous, long-term process, which may/may not include screenings. Screenings are targeted systematic actions at a single point in time that are designed to identify a preclinical condition or disease in individuals suspected of having or being at risk for the specific health impairment. Screening is recommended when the individual will benefit from early treatment or intervention and are part of secondary prevention measures. Universal screening is conducted on all children at defined time intervals or ages, whereas selective screening is conducted only on those children for whom a risk assessment suggests follow-up. Specific details about health supervision for each pediatric age group are included in Unit II.
A variety of measurements are obtained, recorded, and plotted to assist PCPs in assessing growth and pubertal development, including length/height, weight, body mass index, head circumference, and sexual maturity ratings. PCPs must know what to measure, how to measure, and what growth charts are best suited for each child. Growth charts are tools that contribute to forming an overall clinical impression of the child being measured; however, they are not intended to be used as a sole diagnostic instrument. Serial measurements are used to assess patterns and identify aberrations. The Centers for Disease Control and Prevention (CDC) recommends that providers use the World Health Organization (WHO) growth charts to monitor growth in infants and children from birth until 2 years of age and the CDC growth charts for children age 2 and older. Of note, the WHO growth charts are normed for length (supine measurement), whereas the CDC growth charts are for height (standing measurement). The occipital frontal (head) circumference (OFC) is normed for measurements on infants and children in an upright position. A complete set of these age- and sex-specific growth charts are located in the Appendix but can also be found, along with guidelines for use, at the CDC websit Understanding the principles of growth, development, and maturation are key to assessing children over time. Growth refers to an increase in number and size of cells, as well as the increased size and weight of the whole or any of its parts. Development is a gradual change and expansion in capabilities, which represents advancement from lower to more advanced stages of complexity. Maturation represents an increase in competence and adaptability. A change in a structure’s complexity is necessary for it to begin functioning or to function at a higher level. There are distinct pediatric growth and development patterns. For example, growth and development have a cephalocaudal (i.e., head- to-toe) and proximodistal (i.e., midline-to-periphery) progression. There is also a distinct pace/focus to growth, with the infant experiencing rapid growth (mostly head); toddler/preschooler experiencing slow growth (mostly trunk); school- age child experiencing slow growth (mostly limbs); and adolescents returning to rapid growth (mostly sexual maturation).
Each child progresses at her/his own pace. At the same time, growth and development occur on a spectrum. This knowledge means there are ranges of normal physical, social, emotional, and cognitive growth during infancy, childhood, and adolescence. One child gains weight quickly but is slower to speak, whereas another will acquire speech early but be slower to walk. Many children progress smoothly, whereas others do so in fits and starts. Remember to view deviations from the expected in the context of the whole child.
Breastfeeding American Dietetic Association recommend breastfeeding exclusively for the first 6 months of life and then continued breastfeeding in combination with other nutrients for at least the first year. One model that can be used to further these goals encourages providers to focus on interventions that (1) support the mother’s self-efficacy to breastfeed, (2) provide lactation support to mother and family, and (3) increase lactation education for both mother and providers a lower risk of nonspecific bacterial infections, necrotizing enterocolitis, acute otitis media in early childhood, asthma, excessive weight gain, type 2 diabetes, and sudden infant death syndrome (SIDS) decreased risk for breast and ovarian cancer (Ross-Cowerdy, 2017). In addition, breastfeeding is associated with short-term and long-term benefits that protect against cardiovascular risks associated with metabolic syndrome type, hypertension, and cardiovascular disease
1. Contra: Infant with classic galactosemia
2. • Maternal diagnosis of human T-cell lymphotropic virus type I or II
3. • Maternal diagnosis of untreated brucellosis
4. • Maternal diagnosis of cancer and treatment
5. • Maternal human immunodeficiency virus (HIV) infection (except in some areas, see WHO recommendations [Box 16.1]; breastfeeding for HIV-infected mothers is not recommended in developed countries)
6. • Herpetic lesions on the mother’s nipples, areolas, or breast (expressed breast milk can be fed to the infant)
7. • Maternal use of cocaine, phencyclidine (PCP), and cannabis
By approximately 20 weeks, the breast is capable of milk production. he infant should be encouraged to go to each breast for at least 10 to 15 minutes of active suckling, although some infants may spend even longer—up to 20 or 30 minutes. The infant’s behavior is much more important during this time than the clock. However, an infant who falls asleep in 5 minutes should be stimulated to continue active suckling. After the first 24 hours, the infant should be going to the breast 8 to 12 times (or every 2 to 3 hours) in 24 hours for approximately 20 to 45 minutes at each feeding. Frequent suckling stimulates milk production and establishes a regular routine. Exclusive feeding at the breast for the first 4 to 6 weeks should be encouraged to ensure the establishment of adequate milk supply and prevent any nipple preference. Parents need to be on alert if their infant sleeps more than 4 to 5 hours at a time or goes to sleep at the breast in 5 minutes. This infant must be actively wakened and stimulated for feeding. Normal newborn infants lose 5% to 10% of their birth weight in the first few days of life. It is helpful for parents to be aware of both the birth and discharge weights. Once the maternal milk volume increases, the infant begins to gain weight in the range of 0.5 to 1 oz/day or 4 to 7 oz/week.
Many breastfed infants have regained their birth weight by 2 weeks, and others may take up to 3 to 4 weeks before return to birth weight is achieved (Paul et al., 2016). Breastfed infants usually double their birth weight by the time they are 4 to 6 months old and triple it by 1 year old. In the first 2 days of life as the volume of breast milk is increasing, the infant may urinate only one to three times in 24 hours. By day 3, the infant should have three or more wet diapers in 24 hours; and by day 4, the infant should have four to six wet diapers per 24 hours.
[Show More]