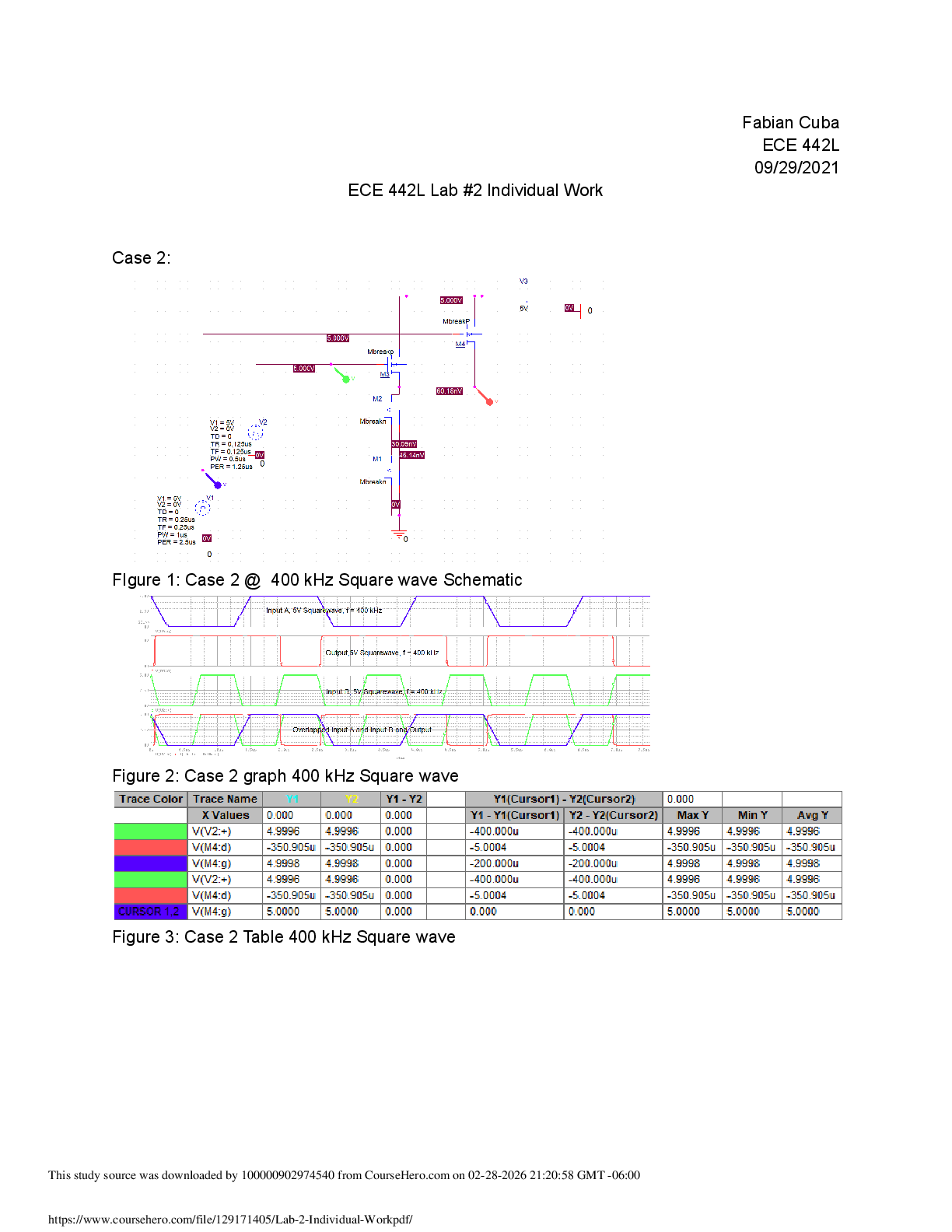
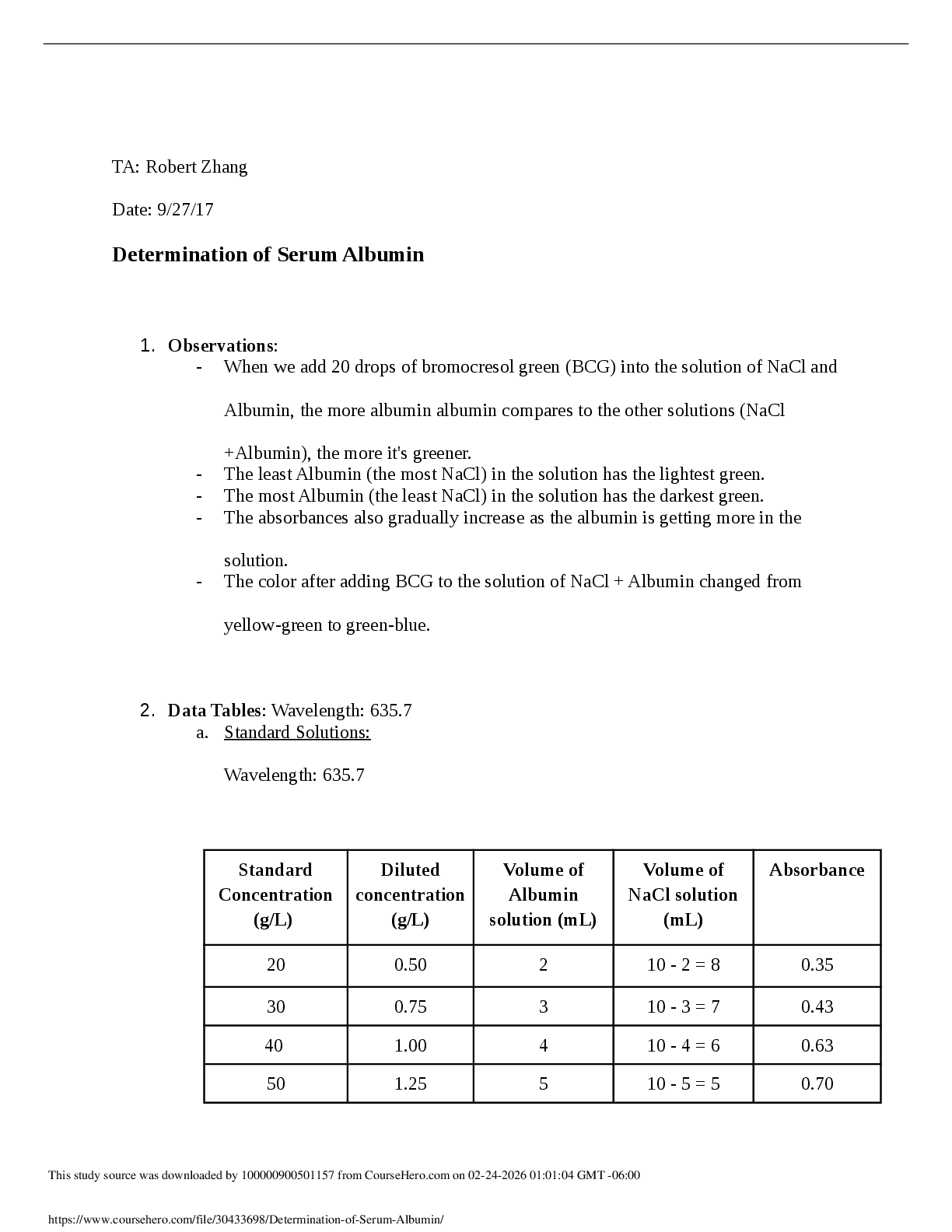
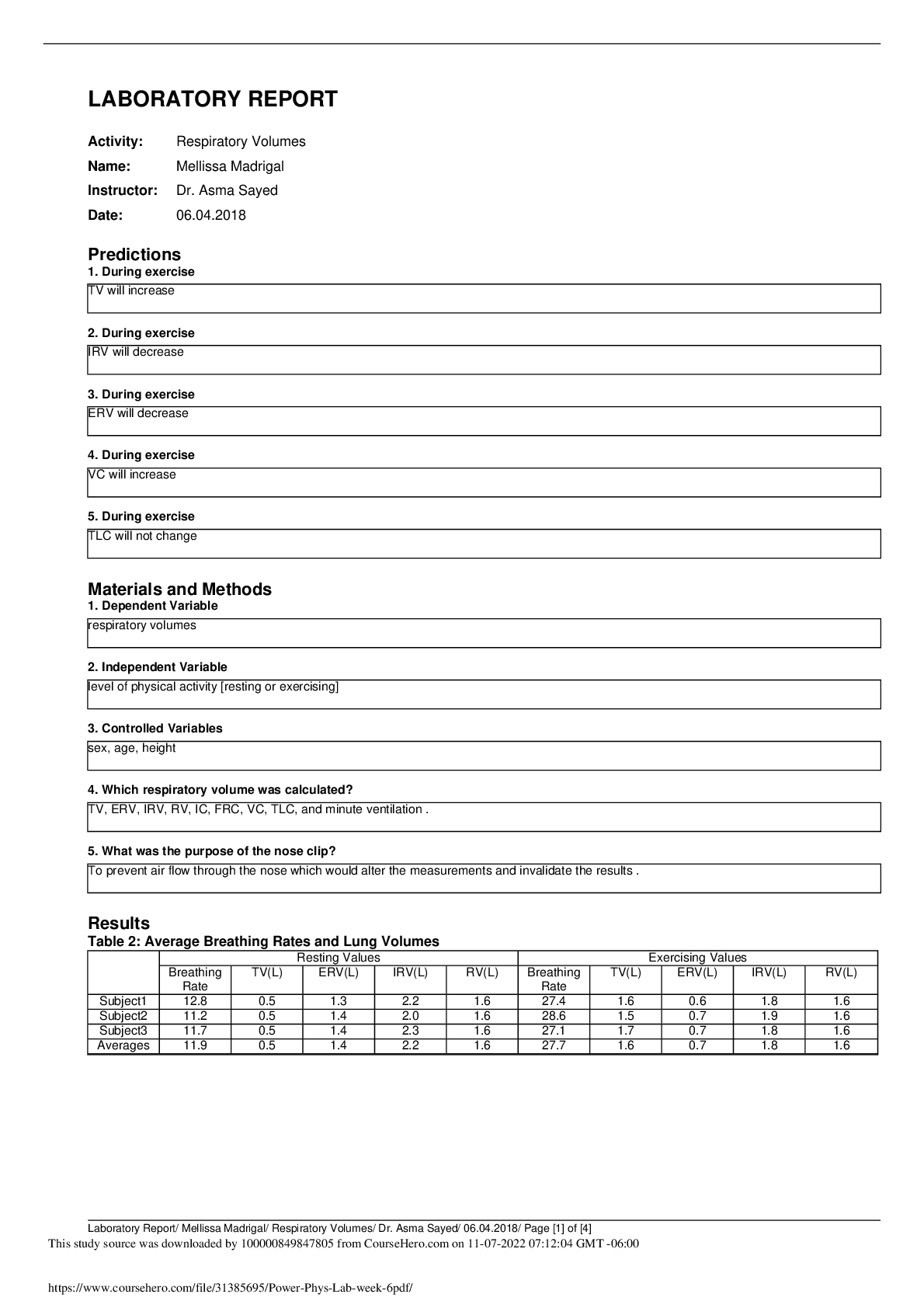
ame: Xavier Thomas Date: 9/4/19 Student Exploration: Periodic Trends Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. On the image at right, the two magnets are the same. Which paper clip would be harder t
...
ame: Xavier Thomas Date: 9/4/19 Student Exploration: Periodic Trends Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. On the image at right, the two magnets are the same. Which paper clip would be harder to remove? 2. Which magnet would be most likely to attract additional paper clips? 3. What is the relationship between the thickness of the book and the ability of the magnet to hold on to and attract paper clips? Gizmo Warm-up Just as the thickness of a book changes how strongly a magnet attracts a paper clip, the size of an atom determines how strongly the nucleus attracts electrons. In the Periodic Trends Gizmo, you will explore this relationship and how it affects the properties of different elements. The atomic radius is a measure of the size of the electron cloud, or the region where electrons can be found. To begin, check that H (hydrogen) is selected in Group 1 on the left. Turn on Show ruler. To measure the radius, drag one end of the ruler to the proton in the nucleus and the other end to the electron. Click Save radius to record the value. 1. What is the radius of hydrogen? Notice that the radius is measured in picometers (pm). A picometer is one trillionth of a meter. 2. On the right side of the Gizmo, select Li. Connect the right side of the ruler to the outermost electron, or valence electron. What is the radius of lithium? | Activity A:Atomic radius | Get the Gizmo ready: · Check that Atomic radius is selected from the drop-down menu. | Question: What factors affect the radius of an atom? 1. Predict: How do you think the radius of an atom will change as you move down a group (vertical column) in the periodic table? 2. Collect data: Use the ruler to measure the atomic radii of the group 1 elements. As you do so, count the energy levels (shown as rings of electrons) in each atom. Record in the table. | Element | H | Li | Na | K | Rb | Cs | Fr | Number of energy levels | | Atomic radius (pm) | 3. Observe: What happens to the radius as you move down group 1? 4. Explore: Turn off Show ruler. Select Li, and then select Be. Observe the radii of the elements in group 2. Then look at other groups. What pattern do you see? 5. Draw a conclusion: In general, what is the effect of the number of energy levels on the radius of an atom? 6. Predict: How do you think the radius of an atom will change as you move across a period (horizontal row) in the periodic table? 7. Collect data: Beginning with Na, record the number of energy levels, number of protons, and atomic radius for each element in period 3.
[Show More]