*NURSING > STUDY GUIDE > N5315 Advanced Pathophysiology Cardiovascular Core Concepts and Objectives with Advanced Organizers. (All)
N5315 Advanced Pathophysiology Cardiovascular Core Concepts and Objectives with Advanced Organizers.
Document Content and Description Below
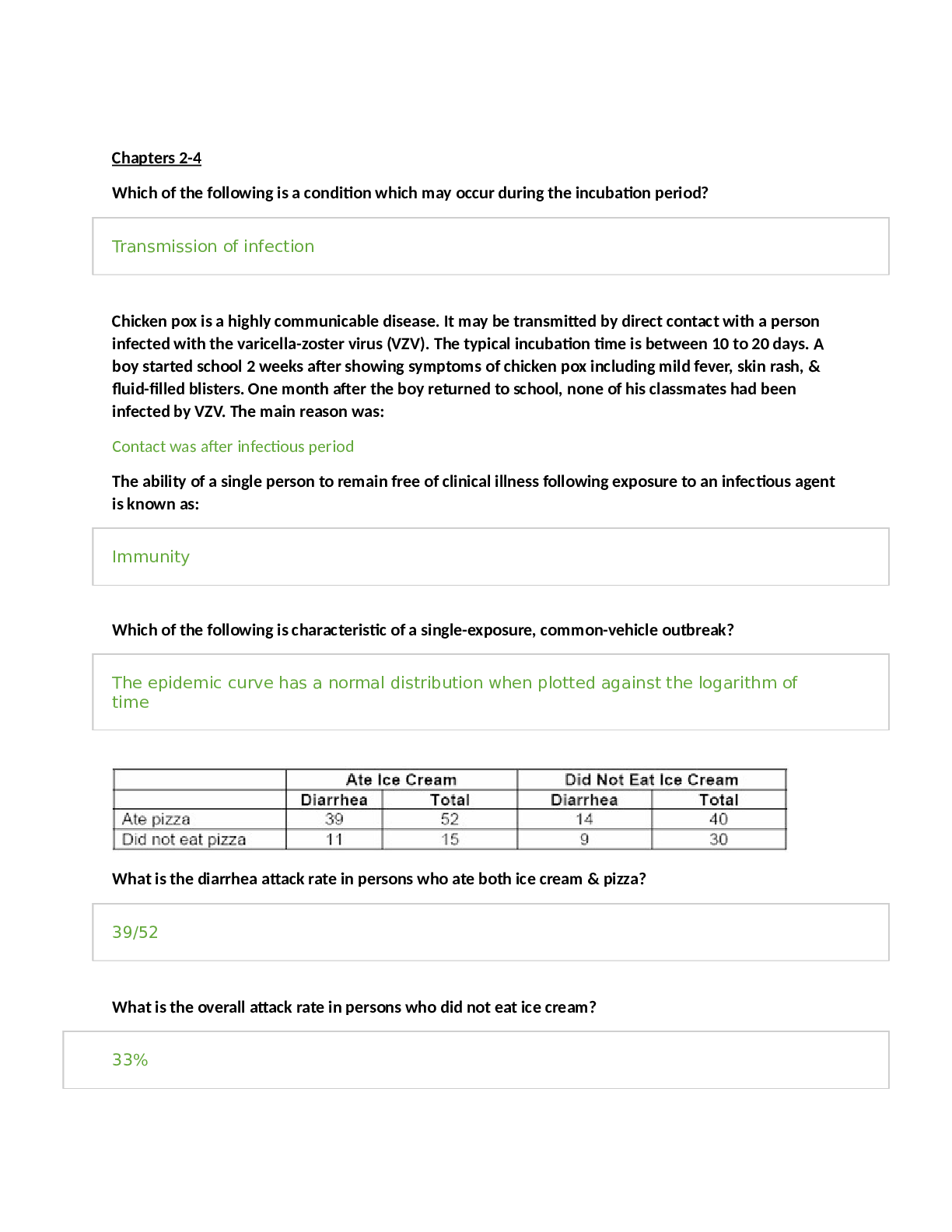
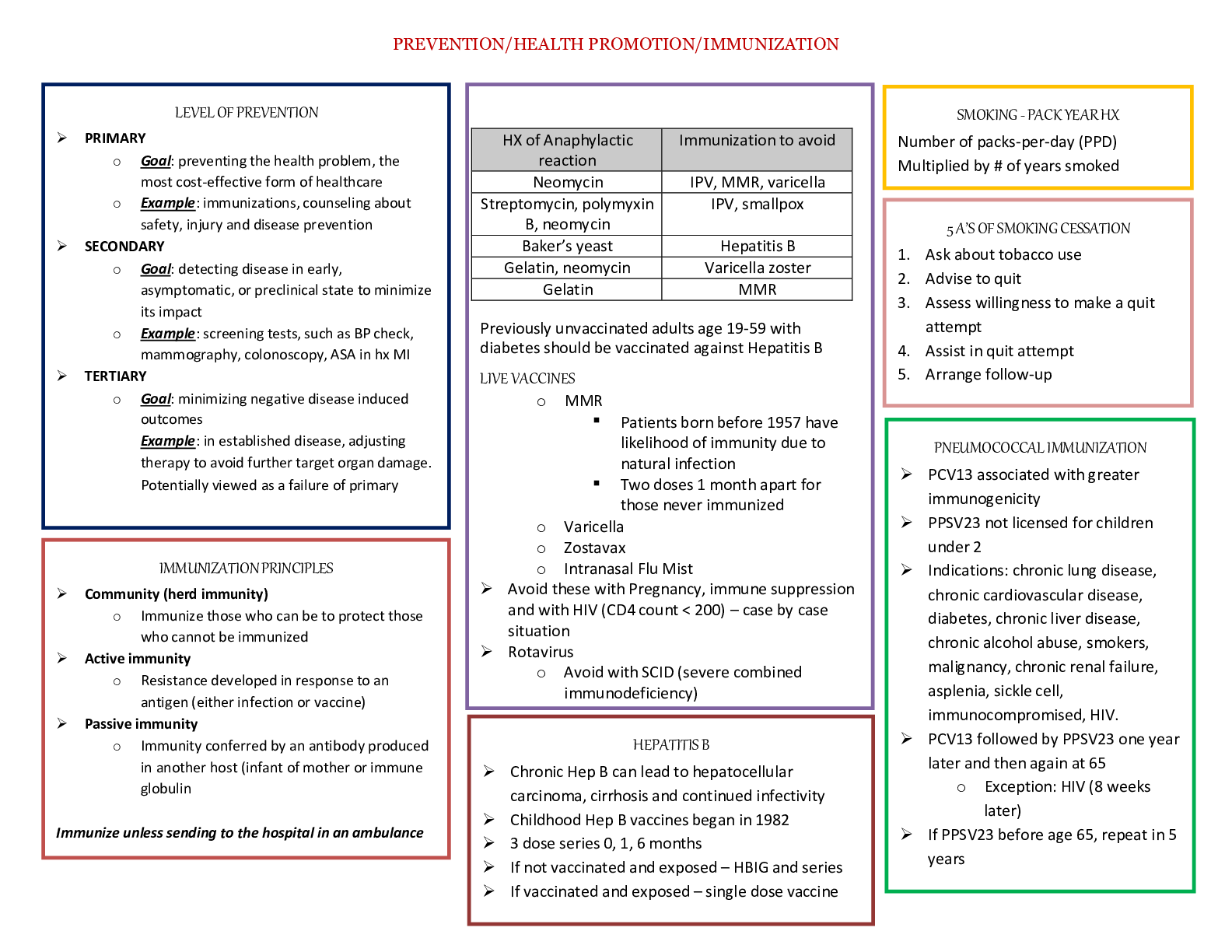
N5315 Advanced Pathophysiology Cardiovascular Core Concepts and Objectives with Advanced Organizers Examine the anatomy and physiology of the cardiovascular system. Cardiovascular Anatomy and Phy... siology I. Explain the cardiac structure and blood flow through the heart chambers/valves. A. The heart is responsible for pumping blood throughout the body to provide the nutrients and oxygen needed for cellular metabolism and life. B. It is divided into four chambers, two atria and two ventricles. 1. The atria lay on top of the ventricles and are receptacles for the blood received from the body or the lungs 2. The ventricles are the larger chambers of the heart that are responsible for the main pumping action of the heart C. The atria and ventricles are separated by a septum which divides the heart into the right and left side. 1. The right atrium receives deoxygenated blood from the body the blood travels from the right atrium, through the tricuspid valve to the right ventricle. From the right ventricle the blood travels through the pulmonic valve, into the pulmonary artery (this is the only artery in the body which carries deoxygenated blood), into the lungs, where it goes to the alveoli and gas exchange occurs. 2. The oxygenated blood then enters the pulmonary vein and is delivered to the left atria from the left atrium it travels through the mitral valve into the left ventriclefrom the left ventricle the blood travels through the aortic valve, into the aorta and goes to the rest of the body to deliver oxygen and nutrients D. The left ventricle is larger than the right because its workload is greater 1. The right ventricle only pumps blood to the lungs whereas the left ventricle has to pump blood to the entire body and has a much higher afterload to push against. 2. The walls thickness of each cardiac chamber depends on the amount of pressure or resistance it must overcome to eject blood 3. Atria have the thinnest walls: low pressure chamber E. The heart has three layers. 1. The endocardium is the innermost layer and is the layer which comes in contact with the blood a. It is made of simple squamous epithelium and underlying connective tissue b. It lines the heart and is continuous with the endothelium that lines arteries, veins, and capillaries, creating a continuous, closed system 2. The middle layer contains the myocytes and is called the myocardium a. The myocardium is the thickest layer composed of cardiac muscle b. The myocytes are responsible for heart contraction 3. The pericardium is the outermost layer and is made up of two layers. a. The fibrous pericardium is made of connective tissue and provides the heart with stability by connecting to the sternum anteriorly and the diaphragm inferiorly. b. Functions: i Prevents displacement of the heart during gravitational acceleration or deceleration ii Acts as a physical barrier that protects the heart against infection and inflammation from the lungs and pleural space iii Contains pain receptors and mechanoreceptors that elicit reflex changes in blood pressure and heart rate c. The serous pericardium has two layers. i The epicardium (visceral layer) lays directly over the heart and contains the coronary arteries. ii The parietal layer is above the epicardium and underneath the fibrous pericardium. d. Pericardial cavity: i Fluid filled space that separates the visceral and parietal pericardia ii Contains about 20 ml of pericardial fluid iii Function: lubricates the membranes that line the pericardial cavity, enabling them to slide over one another with minimum friction as the heart beats F. Valves of the heart 1. One-way heart valves 2. Ventricular relaxation: the two AV valves open and blood flows from the relatively higher pressure in the atria to the lower pressure of the relaxed ventricles 3. After ventricular contraction and ejection, intraventricular pressure falls and the pulmonic and aortic semilunar valves close preventing back flow into the ventricles 4. Right AV valve: tricuspid 5. Left AV valve: mitral valve II. Describe which coronary arteries provide blood to which part of the heart. A. Coronary arteries receive blood through openings in the aorta and cardiac veins empty in the right atrium through the opening of a large vein called the coronary sinus B. The oxygen supply to the myocardium is delivered exclusively by the coronary arteries with 70% to 75% of the oxygen delivered used immediately by the cardiac muscle, leaving little O2 to reserve C. The coronary arteries branch off the aortic root just distal to the aortic valve D. Two main coronary arteries branch off of the aorta: 1. Left Coronary Artery: branches off into the a. Left anterior descending artery: supplied blood to the anterior 2/3rd of the interventricular septum, papillary muscles, and the anterior surface of the left ventricle b. Left marginal artery (obtuse marginal artery): supplies blood to the left ventricle, mostly the anterior side of the left ventricle c. Left circumflex artery: supplied blood to the lateral and posterior walls of the left ventricle and the left atrium 2. Right Coronary Artery: branches off into a. Right coronary artery: supplies blood to the SA and AV nodes in the right atrium and both ventricles (more to right ventricle) b. Right marginal artery: supplies blood to the upper anterior and posterior portions of the right ventricle c. Posterior descending artery (aka interventricular artery): supplies blood to the posterior 1/3 of the interventricular septum, the posterior wall of both ventricles, the posteromedial papillary muscles E. Coronary arterial blood flow may be right dominant or left dominant with most people being right dominant (around 85%) 1. Right dominant: the posterior descending artery arises from the right coronary artery 2. Left dominant: the posterior descending artery arises from the left circumflex artery (8%) 3. Codominant: the posterior descending artery arises from both the left and the right coronary arteries (7%) F. Collateral arteries: connections or anastomoses between the branches of the same coronary artery with branches of the left 1. Common within the intraventricular and interatrial septa, the apex of the heart and over the anterior surface of the R ventricle, around the SA node 2. Gradual coronary occlusion results in the growth of coronary collaterals G. Coronary artery occlusion most commonly occurs in the left anterior descending artery, followed by the right coronary artery and the left circumflex artery 1. The left atria is the most posterior portion of the heart 2. When it enlarges, it can cause dysphagia by compression on the esophagus of can lead to hoarseness from compression of the left recurrent laryngeal nerve which is a branch of the vagus nerve III. Conduction: A. Sympathetic and parasympathetic nerves affect heart rate 1. Sympathetic: increases heart rate and conduction through the nodes 2. Parasympathetic: slows heart rate and prolongs intermodal conduction time 3. Both cause coronary vasodilation B. Heart beats in the absence of nervous connection; mediated by the physical needs to the body and the nodes C. Electrical impulses are normally generated from the SA node (because it has the fastest rate of depolarization”, located in the right atrium 1. SA node generates impulses around 60-100 action potentials per minute, 60-100 bpm 2. Impulses generated from the SA node are transmitted to the AV node then to the Bundle of His and finally through the bundle branches of the Purkinje fibers D. The atrial node is located in the right atria wall just above the tricuspid valve, it mediates how fast impulses are transmitted to the ventricles 1. If the SA node fails, the AV node will generate an impulse 2. It can generate 40-60 action potentials per minute 3. AV node will pass the impulse down the conduction system to the bundle of His E. Bundle of His (right and left branches) 1. Right bundle branch (RBB): branches to the right ventricular apex 2. Left bundle branch (LBB): branches to the anterior and posterior left ventricular wall 3. If the AV node fails, will generate an action potential of less than 40 beats per minute F. The impulse will then travel to the purkinje fibers, which travel up the wall of the ventricles and cause contraction of the ventricles IV. Analyze the process of cardiac action potentials. A. Automaticity: the property of generating spontaneous depolarization to threshold, enable the SA and AV nodes to generate cardiac action potential without any stimulus. The ionic basis for diastolic depolarization is the regular rhythmic oscillation of calcium with the automatic cells, “calcium clock” B. Ventricular Action Potentials 1. Generated by the bundle of His or the purkinje fibers 2. Occur in five phases: cardiac cells begin with a resting membrane potential of -85mV a. Phase 0: Rapid depolarization. Na+ influx occurs as a result of the voltage gated Na+ channels opening b. Phase 1: Initial repolarization of the cells. The voltage gated Na+ channels are closed and the voltage gated K+ channels begin to open and K+ begins to leave the cell slowly. c. Phase 2: Plateau phase. The voltage gated Ca+ channels open which allows for the influx of Ca+ into the cells. The influx balances out the potassium efflux thus causing a temporary plateau in the repolarization. The influx of Ca triggers the release of more Ca from the sarcoplasmic reticulum and thus causes myocardial contraction. d. Phase 3: Rapid repolarization with massive K+ efflux. The voltage gated K+ channels open and the voltage gated Ca+ channels close causing rapid repolarization. e. Phase 4: Resting potential of -85mV. High K+ permeability from the K+ channels C. SA and AV node Action Potentials 1. Generate action potential slightly different than the bundle of His and purkinje fibers 2. Action potentials do not undergo phase 1 or phase 2 3. Occurs in three phases: a. Phase 0: Depolarization, which results from the opening of the voltage gated Ca+ channels. The voltage gated Na+ channels are inactivated because the resting membrane potential is less negative than the ventricle. This results in a slow conduction of the impulse which is used by the AV node to prolong transmission from the atria to the ventricles. b. Phase 3: Repolarization which results from closure of the voltage gated Ca+ channels and the opening of the voltage gated K+ channels, causing K influx c. Phase 4: Slow depolarization from the slow, spontaneous influx of Na+. This is what gives the SA and Av nodes the property of automaticity, they do not need any stimulus to generate an action potential, done automatically 4. The slow inward current, mediated by calcium through transient and long-lasting channels and sodium ions flowing through “slow channels” of the cell membrane are responsible for the action potential of the SA and SV node, blocking Ca can have profound effects on this current and alter the heart rate 5. Acetylcholine and the drug adenosine cause a decrease in the rate of depolarization and decrease heart rate (decrease heart rate, slows conduction through the AV nodes, and reduces myocardial contraction strength) 6. Catecholamines increase the rate of depolarization and increase the heart rate V. Discuss how potassium and calcium imbalances affect myocardial action potentials, contraction, and the clinical manifestations which result. A. Calcium: 1. Hypocalcemia: increased neuromuscular excitability, prolonged ventricular depolarization and decrease cardiac contractility seen with prolonged QT interval and risk of cardiac arrest 2. Hypercalcemia: decreased excitiability, bradycardia and varying degrees of heart block, dysrhythmias B. Potassium 1. Hypokalemia: increased excitability; dysrhythmias, weak, irregular pulses, ventricular fibrillation, cardiac arrest 2. Hyperkalemia: decreased excitability; dysrhythmias, bradycardia, heart block, cardiac arrest VI. Discuss the effect of a magnesium imbalance on the cardiovascular system. A. Magnesium is required for the binding of ATP, necessary for the metabolism of chemical energy for myocardial contraction B. Hypomagnesemia: tachycardia, hypotension 1. Associated with insulin resistance in diabetes and left ventricular hypertrophy 2. Magnesium supplements have several benefits including improved myocardial metabolism and cell function, improved vascular smooth muscle tone and PVR, afterload, and cardiac output, and reduced cardiac dysrhythmia C. Hypermagnesemia: hypotension, bradycardia, heart block, cardiac arrest VII. Electrocardiogram: A. P wave: represents atrial depolarization and is located just before every QRS complex; atrial repolarization cannot be seen in the EKG because it is hidden by the QRS complex B. PR interval: represents the conduction delay through the AV node, usually 0.12-0.20 seconds long; the time from the onset of atrial activation to the onset of ventricular activation C. QRS complex: Represent ventricular depolarization 1. Q wave is the first negative projection 2. R wave is the first positive projection 3. S wave is the second negative projection 4. Normally <120 miliseconds long D. QT interval: Mechanical contraction of the ventricles, the “electrical systole” of the ventricles 1. Prolonged: occurs with a number of drugs such as fluoroquinolones, Diflucan, Zofran, many psychiatric medication, magnesium deficiency 2. When prolonged increases the risk for ventricular arrhythmias such as torsades, V-tach or V-fib E. T wave: first positive projection after the QRS. Represent ventricular repolarization. F. ST segment: represents ventricular repolarization, when the entire ventricular myocardium is depolarized G. U wave: if present will be a second positive projection after the QRS complex. It may be present with bradycardia or hypokalemia VIII. Explain the difference between cardiac hemodynamic measures: Cardiac output, stroke volume, ejection fraction, preload and afterload. A. Cardiac output: 1. Volume of blood flowing through either the systemic or pulmonary circuit; expressed in liters per minute 2. Calculated by multiplying HR x SV 3. Normal cardiac output at rest is about 5 L/min 4. Four factors affect cardiac output directly: a. Preload: pressure generated at the end of diastole b. Afterload: resistance to ejection during systole c. Myocardial contractility d. Heart rate 5. Decrease in CO, indicated heart failure B. Stroke volume: volume of blood ejected during systole 1. Normal: 60-100 ml/beat 2. Depends on the force of contraction, determined by three factors a. Changes in the stretching of the ventricular myocardium caused by variations in ventricular volume (preload) b. Alterations in nervous system input to the ventricles c. Adequacy of myocardial O2 supply 3. Chemicals effecting contractility: inotropic agents a. Positive: most important are epinephrine and norepinephrine; also dopamine b. Negative: most important acetylcholine 4. Decrease in SV indicates heart failure C. Ejection fraction: 1. The amount of blood ejected with each heart beat: ventricles do not eject all of the blood they contain with each heartbeat 2. Calculated by dividing the SV by the end-diastolic volume (EDV): SV/EDV 3. End-diastolic volume: normally 70-80 ml/m2 4. Normal ejection fraction: 55% or higher 5. Ejection fraction is a clinical indicator of ventricular function IX. Analyze factors which affect cardiac contractility. A. Explain the normal function of myocardial cells. 1. Similar in appearance to skeletal muscle cells but have key difference that allow cardiac cells to: a. Transmit action potentials quickly from cell to cell: intercalated disks b. Maintain high levels of energy synthesis: contain large number of mitochondria c. Gain access to more ions, particularly sodium and potassium: T tubules d. Formed in sarcomeres to allow for stretch during diastole B. Explain the process of cardiac contraction and relaxation. 1. The degree of tension, which is produced by the LVEDV aka preload, of the left ventricle and the amount of intracellular calcium are responsible for contraction a. The greater the tension, the stronger the contraction b. The greater amount of intracellular calcium, the stronger the contraction 2. Calcium interacts with troponin C, which causes tropomyosin to move thus allowing actin and myosin to work together to cause contraction 3. The reverse is true: myocardial relaxation occurs when intracellular calcium levels decrease, drugs which alter intracellular calcium levels will affect tension and contraction C. Autonomic Nervous system 1. Autonomic nervous system divided into the sympathetic and parasympathetic systems. Each have a distinct effect on the CV system a. Sympathetic: increase HR b. Parasympathetic: decreased HR 2. Epinephrine and Norepinephrine are neurotransmitters and hormones released by the sympathetic nervous system and the adrenal gland 3. Acetylcholine is released by the parasympathetic nervous system and decreased heart rate, contractility, and causes vasodilation 4. Chronotrophy: refers to heart rate 5. Inotrophy: refers to contraction D. Main adrenergic receptors which affect the CV system Receptor Location Effect Beta 1 Heart, the conduction system Increase contractility, heart rate, and renin secretion Chronotropy and inotropy Beta 2 Mainly in the blood vessels Smooth muscle of the bronchi Vasodilation Increases coronary blood flow Located in the smooth muscle of the bronchi and when stimulated cause bronchial dilation; hence administration of albuterol during periods of respiratory distress Alpha 1 Blood vessels Vasoconstriction Dopamine Renal, mesenteric, coronary, and cerebrovascular blood vessels Vasodilation E. Evaluate the effects of the catecholamines on the cardiovascular system Catecholamine Effect on the CV system Epinephrine Released by the adrenal medulla and reaches the heart through the bloodstream Primarily stimulates the heart’s beta 1 receptors: Increase myocardial contractility and heart rate Dilates the vessels of the liver Beta receptors: Overall effect is an increased influx of Ca during the action potential plateau that increases the contractile strength of the heart Simultaneous effects on both beta 2 and alpha 1: results in a cancellation of their individual functions and thus the vascular tone does not change Main purpose as a drug is to increase contractility When administered in high doses its alpha 1 effects override the beta 2 effects: causing vasoconstriction and increased systemic vascular resistance while still enhancing myocardial contractility and cardiac output Vasoconstriction except in liver, coronary, or skeletal muscles Norepinephrin e Released by the sympathetic nervous system Stimulates both the beta 1 and alpha 1 receptors Increases myocardial contractility and causes vasoconstriction Basis for the drug norepinephrine (Levophed) Dopamine Administered at low doses: stimulate dopamine 1 receptors and causes vasodilation Moderate doses: stimulates the beta 1 receptors and improves myocardial contractility and cardiac output High doses: alpha 1 activity and causes vasoconstriction Dobutamine Stimulates the beta 1 receptors in the myocardium and improves contractility and increases heart rate F. Describe pathologic and physiologic factors which increase or decrease myocardial contraction. Factors which Increase Cardiac Contraction Factors which Decrease Cardiac Contraction Catecholamines: increase the activity of the calcium pump in the sarcoplasmic reticulum increasing the release of Ca Beta blockers: block the effects of catecholamines Increases in intracellular Ca Heart failure with systolic dysfunction Decreased extracellular sodium: decreases the activity of the Na/Ca exchanger Acidosis Digitalis Hypoxia/hypercapnia Thyroid hormone Nondihydopyridine calcium channel blockers Acetylcholine released by the vagus nerve G. Cardiac Action Potentials are the basis for anti-arrhythmic drugs. Four classes: 1. Class One: block sodium channels 2. Class Two: inhibit the B-adrenergic stimulation, aka beta blockers 3. Class Three: prolong the action potential duration by blocking potassium influx 4. Class Four: block the cardiac calcium current, aka calcium channel blockers H. Describe how diltiazem, verapamil, digitalis, beta blockers and dobutamine affect myocardial contraction. Medication Increases or decreases contraction How does it increase or decrease contraction? Diltiazem & Verapamil Decreases Negative inotrophic effect Non-dihydropyridine calcium channel blockers Inhibit the influx of calcium into the myocardium Decreasing CA concentration which in turn decreases myocardial tension and contractility Should be avoided in persons with systolic heart failure Major effect of these medications is to decrease the strength of cardiac contraction Also modify the pacemaker activity so that myocardial demand is reduced Digitalis Increases Positive inotrophic effect Blocks the Na/K pump which increases intracellular Na, decreases the activity of Na/Ca exchanger and increases intracellular Ca Beta Blockers Decrease Negative inotrophic effect Decrease catecholamine-induced elevations of heart rate, myocardial contractility, and blood pressure Reduction in heart rate provides additional diastolic filling time for coronary perfusion, leading to enhanced O2 delivery to the heart Dobutamine Increase Positive inotrophic effect Increase contractility and can help raise BP in hypotensive individuals I. Baroreceptor reflex: 1. Senses decreased BP and increased heart rate and contractility, and causes vasoconstriction of arteries 2. Sense increased BP and cause stimulation of the parasympathetic system and inhibition of the sympathetic system to decrease BP, HR, and contractility Aging and the Cardiovascular System I. Describe how the normal anatomy and physiology of the arteries and left ventricle are altered by the aging process. A. Arteries: 1. Arterial stiffening, an important contributor to hypertension 2. Stiffening d/t age-related changes in cross-linking of collagen, and increase in the amount of collagen, deposition of calcium, and changes in elastin B. Left Ventricle: 1. Left ventricular hypertrophy and fibrosis 2. Ventricle must work harder as the arterial system becomes stiffer 3. Increased risk for Valvular disease, heart failure related to stiffness of the L ventricle I. Explain the prenatal and post-natal development of the cardiovascular system. II. Differentiate between the effects of aging on the anatomical and physiologic processes of the cardiovascular system. Cardiovascular structure / Process Effects of aging Cardiac Index Resting: Unchanged or slightly decreased in women only Exercise: declines because of a decrease in heart rate and stroke volume Heart Rate Decreases at rest and exercise, possibly because of decreased cardiovascular response to catecholamines Stroke Volume Slight increase at rest and exercise Afterload Increases at rest End Diastolic Volume Unchanged with aging End Systolic Volume Unchanged with rest but higher with exercise with increased age Contraction Resting: Increased because of prolonged relaxation Exercise: decreases with vigorous exercise Arteries Arterial stiffening, an important contributor to hypertension Stiffening d/t age-related changes in cross-linking of collagen, and increase in the amount of collagen, deposition of calcium, and changes in elastin Left Ventricle Left ventricular hypertrophy and fibrosis Ventricle must work harder as the arterial system becomes stiffer Analyze the etiology, pathophysiology and clinical manifestations of disorders which affect the cardiovascular system. Vascular Disease I. Blood Vessel Anatomy A. Oxygenated blood: arteries arterioles capillaries where nutrient exchange between the blood and tissues occurs B. Deoxygenated blood: venules veins heart C. Each blood vessel has three layers 1. Tunica Intima: the innermost layer a. It is the layer which comes in direct contact with the blood b. Made up of squamous epithelial cells, a layer of connective tissues, and a basement membrane c. The squamous cells have a role in coagulation, antithrombogenesis, fibrinolysis, immune function, tissue growth and healing, vasoconstriction, and relaxation 2. Tunica Medica: made up of smooth muscle, assists with the contraction and relaxation of the artery (larger in arteries) 3. Tunica Externa: outermost layer and is made of connective tissue, nerves, and lymphatic vessels II. Assess the etiology, clinical manifestations, and the pathophysiology of the diseases which affect the blood vessels. Disease Etiology/ Clinical Manifestations Pathophysiology Hypertension ● Primary ● Secondary ● Malignant Hypertension: sustained systolic > 140 and diastolic >90 Primary: -No known cause, genetic factors -Risk factors: family hx, advancing age, gender (men 55 y/o, women 77 y/o), black race, high dietary sodium, glucose intolerance, cigarette smoking (nicotine causes vasoconstriction), obesity, alcoholism, low dietary potassium, calcium, or magnesium HTN: Caused by increases in cardiac output or total peripheral resistance or both Primary: results from a complicated interaction between genetics and the environment insulin resistance and dysfunction of the SNS, RAAS, and natriuretic hormones renal salt and water retention and vasoconstriction increased blood volume and increased peripheral resistance sustained hypertension Sustained inflammation also contributed to renal dysfunction that leads to dysfunction of RAAS and increased sodium and water retention Overactivity of the SNS increased HR, insulin resistance, vascular remodeling, procoagulant effects endothelial dysfunction and narrowing of -ACE inhibitors to treat Secondary: caused by altered hemodynamics associated with an underlying primary disease that raises vascular resistance or CO HTN: no clinical manifestations in the early stages; eventually evidence of heart disease, renal insufficiency, CNS dysfunction, impaired vision, impaired mobility, vascular occlusion, or edema Assessment for HTN should include: examination of optic fundi, calculation of BMI, auscultation for carotid, abdominal, or femoral bruit, examination of heart and lungs, palpation of the abdomen, assessment of lower extremity pulses and edema, and neurologic examination, urinalysis vessels and vasospasms HTN Decreased production of vasodilators: iNO HTN increases risk for target organ disease events of the kidney, brain, heart, extremities, and eyes -CV: left ventricular hypertrophy (diastolic heart failure, muscle replaced with collagen), angina, CHF, CAD, MI, and sudden death -Vascular: aneurysms, intermittent claudication, gangrene from vessel occlusion -Renal: parenchymal damage, nephrosclerosis, renal arteriosclerosis, and renal insufficiency or failure (microalbuminuria: early sign of impending renal dysfunction) -Eyes: retinal sclerosis, exudation, and hemorrhage -Neuro: transient ischemia, stroke, cerebral thrombosis, aneurysm, and hemorrhage Malignant: rapidly progressive HTN, diastolic >140mmHg that can cause encephalopathy because of inability to regulate blood flow to cerebral capillaries d/t high arterial pressurescapillary permeability increases from high hydrostatic pressure in capillaries vascular fluid exudes to the interstitial space cerebral edema and dysfunction if not reversed Atherosclerosis Injury to the arterial wall: -Hypertension: which causes endothelial cell damage -Diabetes: associated with increased lipids, HTN, and abnormalities with coagulation, platelet adhesion/aggregation, increased oxidative stress, and functional changes in the endothelium -Cigarette smoking also causes damage to the vessel wall. Arteriosclerosis: Broad term used to describe the thickening and loss of elasticity of the arterial walls Pathologically, lesions progress from endothelial injury and dysfunction to fatty streak to fibrotic plague to complicated lesion Three types: (1)Monckeberg: calcification of the medium-sized arterial walls and most commonly affects the radial and arterial arteries. Does not obstruct flow because -Hyperlipidemia: process can affect any artery in the body. It is the leading cause of coronary artery disease, most commonly affects the proximal portions of the coronary arteries, the larger branches of the carotid arteries, the circle of Willis, the large vessels of the lower extremities, the renal and mesenteric arteries Clinical manifestation: symptoms and signs that result from inadequate tissue perfusion because of obstruction of vessels Complications: coronary artery disease, myocardial infarction, carotid artery disease, cerebral vascular disease, stroke, mesenteria ischemia, peripheral vascular disease, and renal artery stenosis the initima is not involved. (2) Arteriolosclerosis: results for hyaline thickening or proliferative changes of the small arteries. Most commonly affects the kidneys as a result of HTN or DM. (3) Atherosclerosis: results from the accumulation of fat, WBC, platelets and other substances in the vessel wall and forms a plague in the intima of the artery which can rupture and cause a myocardial infarction The initial step of this process is arterial wall injury (1) Fatty streak: Once the epithelial injury occurs and causes the endothelial cells to become inflamed, they then lack the ability to produce sufficient amounts of antithrombotic and vasodilating cytokines, macrophages and platelets adhere to the area of injury in the endothelium and more toxic oxygen radicals are released by the macrophages Platelets further stimulate the inflammatory response. The toxic oxygen radicals create oxidative stress and oxidize LDL. This causes additional endothelial damage. -Growth factors, angiotensin II, fibroblast growth factor, TGF-β, and platelet derived growth factor, are released which cause smooth muscle hyperplasia -LDL penetrates the subintima of the vessel and becomes trapped and is oxidized by the above process Oxidized LDL is toxic and causes smooth muscle proliferation (hyperplasia) and increases endothelial adhesion molecule expression resulting in the adhesion of more macrophages which penetrate the vessel wall macrophages then engulf the LDL, and they are then known as foam cells accumulation of the foam cells (foamy macrophage ingesting lipids) increases over time and causes a fatty streak the fatty streak produces more toxic oxygen radicals, triggers more of an immune and inflammatory response all of which causes ongoing, progressive damage to the vessel (2) Fibrous plague: Smooth muscle hyperplasia is ongoing, produces collagen and migrates over the fatty streak to form a fibrous plaque This plaque may calcify, protrude in the vessel lumen and occlude flow (3) Complicated Lesion: Plaques can be unstable and may rupture secondary to inflammatory activations and acutely occlude blood flow and result in ischemia or infarction. Peripheral Arterial Disease Risk factors: 65 y/o or older, blacks, same risk factors as atherosclerosis PAD is a significant predictor of systemic atherosclerotic disease Refers to atherosclerotic disease of the arteries that perfuse the limbs, especially the lower extremities Lower-extremity ischemia can be gradual or acute Gradually increasing obstruction to arterial blood flow to the legs in the iliofemoral vessels results in pain with ambulation: intermittent claudication If thrombus forms over atherosclerotic lesion, perfusion can cease acutely and lead to severe pain, loss of pulses, and skin color changes in the effected extremity Coronary Artery Disease and Acute Coronary Syndrome I. Examine the risk factors, etiology, clinical manifestations, pathophysiology and consequences of coronary artery disease and acute coronary syndrome. A. Coronary Artery Disease: 1. Caused by atherosclerosis which occurs in the coronary arteries 2. CAD ultimately leads to lumen narrowing and may cause acute ischemia which may be relieved or progress to a myocardial infarction 3. Ischemia: local state in which cells are deprived of blood supply, still alive and functioning but cannot function normally 4. Chronic ischemia: may occur with or without an infarction and lead to cellular damage and cause heart failure B. Explain how modifiable risk factors contribute to the development of coronary artery disease: Risk Factor Effects Dyslipidemia An increased level of LDLs is a very strong predictor of CAD and future coronary events Decreased HDL cholesterol is a strong indicator of coronary risk Hypertension Causes endothelial injury, causes myocardial hypertrophy, which increases myocardial demand for coronary flow Cigarette Smoking Nicotine causes the release of catecholamines, which in turn cause vasoconstriction and high blood pressure. As BP increases so does cardiac workload and O2 demand Smoking associated with increased LDL and decreased HDL, and contributes to vessel inflammation and thrombosis Diabetes Mellitus Causes endothelial damage, thickening of blood vessel walls, increased inflammation and leukocyte adhesion to the vessel walls, increased thrombosis and decreased production of nitric acid, a vasodilating substance Also associated with dyslipidemia Obesity and Sedentary Lifestyle Metabolic syndrome: obesity, dyslipidemia, and hypertension Increased CAD risk related to insulin resistance, decreased HDL, increased BP and inflammation Sedentary lifestyle increases risk of obesity, physical activity and weight loss offer substantial reductions in risk factors of CAD C. Describe the function of VLDL, LDL, HDL, triglycerides, and cholesterol, and the impact this has on the diagnosis and treatment of patients with hyperlipidemia. Lipoprotein Function VLDLs Primarily triglycerides and protein LDLs Cholesterol and protein Responsible for the delivery of cholesterol to the tissues LDLs are a key player in the development of atherosclerosis: High dietary intake of cholesterol and fat/ genetic predisposition leads to accumulation of LDLs LDL oxidation in the vessel walls, and phagocytosis by macrophages are key steps in the pathogenesis of atherosclerosis LDL also play a role in endothelial injury, inflammation, and immune responses HDLs Phospholipids and protein Responsible for “reverse cholesterol transport” which returns excess cholesterol from the tissues to the liver, where it binds to hepatic receptors and is processed and eliminated as bile or converted to cholesterol-containing steroids Remove excess cholesterol from the arterial wall through several pathways including mediating the efflux of cholesterol from “foam cells” Participate in endothelial repair and decrease thrombosis The effectiveness of drugs that increase HDLs in the prevention of CAD are being evaluated Triglycerides Elevated serum VLDLs Associated with increased risk of CAD, especially in combination with other risk factors Total Cholesterol Because triglycerides increase risk of CAD, non-HDL cholesterol is used to assess CV risk rather than just LDL D. Assess the use of troponin I for cardiac risk assessment and diagnosis of ACS. 1. Troponins are a very reliable way to diagnose myocardial infarction a. Can be detected in 2-4 hours of onset of the pain and should be monitored b. They peak in 24 hours and disappear within 7-10 days c. EKG needed to specify type 2. Can be used to estimate infarct size and therefore likelihood of complications 3. Can be used to assess for risk for future CHD events, mortality, and heart failure E. Explain the pathological steps of myocardial ischemia and myocardial infarction. 1. Etiology: Narrowing of a major coronary artery by more than 50% impairs blood flow sufficiently to hamper cellular metabolism under conditions of increased myocardial demand: a. Increased demand: Tachycardia, exercise, hypertension (hypertrophy), and Valvular disease; coronary spasm, hypotension, dysrhythmias, and decreased O2 carrying capacity (anemia, hypoxemia) b. Most common cause of decreased coronary blood flow is atherosclerotic plaques in the coronary circulation 2. Imbalance between coronary supply and myocardial demand myocardial O2 deficiency Myocardial ischemia: less than 20 minute attack leading to abnormal responses to electrical impulses and dysrhythmias Myocardial infarction: greater than 20 minutes leading to lack of response to electrical impulses and failure of heart to contract 3. Cellular injury is a result of ongoing hypoxia 4. After 10 seconds of occluded flow, the myocytes become cyanotic oxygen reserves are used up glycogen stores decrease and glycolysis is not able to supply all the needed energy to the heart and this leads to less ATP production hydrogen ions and lactic acid accumulate from the anaerobic metabolism potassium, calcium, and magnesium are lost from the cells myocardial cells deprived of nutrients lose contractility and result in a diminished contraction 5. Ischemic cells release catecholamines which further stresses the heart and increases the risk of arrhythmias and heart failure a. Norepinephrine decreased insulin secretion and hyperglycemia occurs b. This can be seen by 72 hours post MI and is associated with increased mortality 6. Reperfusion injury can be caused when blood flow is restored: This triggers the release of toxic oxygen radicals, calcium influx, and pH changes that cause persistently open mitochondrial permeability transition pores and contributes to cellular death 7. Angiotensin II is released during an MI and causes vasoconstriction, thereby increasing afterload and myocardial workload 8. Cardiac cells remain viable for up to 20 minutes 9. Necrosis occurs in 20 minutes: Coagulative necrosis a. Area of necrosis will determine the function of the heart and clinical manifestations b. If MI occurs in the RCA, the SA node may be affected 10. Myocardial Infarction: a. When coronary blood flow is interrupted for an extended period, myocyte necrosis occurs b. Thrombus occludes vessel ischemia necrosis and death c. Duration of ischemia determines the size and character of infarction d. Clinical manifestations: i. Sudden, severe chest pain with radiation to the neck, jaw, back, shoulder, or left arm is common ii. Nausea and vomiting iii. Temporary increase in HR and BP hypotension iv. Abnormal extra heart sounds, cardiac murmurs v. Pulmonary congestion vi. Peripheral vasoconstriction: skin cool and clammy F. Describe those persons most at risk for, the etiology of, and the presentation (clinical manifestations) of stable, prinzmetal angina and silent ischemia and the implications this has for patient education, risk reduction and treatment. Type Clinical Manifestations Etiology Stable Angina CAD is the basis for stable angina Caused by myocardial ischemia Chest pain is transient in nature and usually is relieved with rest Angina pectoris: transient chest Chest pain caused ischemia Ischemia is caused by the gradual lumen narrowing and is present when the myocardial oxygen demand is increased, typically during times of activity discomfort, ranging from heaviness to pressure feeling Women: atypical chest pain, palpitations, sense of unease, severe fatigue Pallor, diaphoresis, and dyspnea may be associated with the pain Lack of relief indicates an individual may be developing infarction With CAD and stable angina, the blood flow at rest is sufficient to meet the myocardial oxygen needs; however, when activity occurs the workload of the heart increased and therefore O2 requirements increase but the blood flow does not resulting in the chest pain from ischemia Prinzmetal Angina Chest pain which occurs at rest, typically at night and may or may not be associated with atherosclerosis and is usually caused by vasospasm Often pain occurs at night during REM sleep and may have cyclic pattern of occurrence Chest pain attributable to transient ischemia of the myocardium that occurs unpredictable and almost exclusively at rest If spasm persists infarction and serious dysrhythmias may occur Usually successfully treated w/ vasodilators Silent Ischemia Nonspecific symptoms, fatigue, dyspnea, or feeling of unease More common in women May occur during mental stress: d/t increased CRP, decreased vasodilator activity, and hypercoagulable state Seen in local nerve damage d/t dysfunction in diabetes, following cardiac surgical interventions Screening based on presence of risk factors and stress imaging Indicator of increased risk for serious CV disease and aggressive tx may be indicated G. Differentiate between the three types of acute coronary syndrome (unstable angina, NSTEMI, STEMI) and their clinical manifestations. H. Acute coronary syndrome: develops when there is a sudden coronary obstruction cause by thrombus formation over a ruptured or ulcerated atherosclerotic plaque 1. Atherosclerotic plague prone to rupture is called “unstable” and has a core of oxidized LDLs and a thin fibrous cap 2. Once unstable can caused ulceration of rupture and thrombosis leading to ACS and possible myocardial infarction Acute Coronary Syndrome Type Pathophysiology Clinical Manifestations Unstable Angina Ischemia is caused by a labile thrombus which does not occlude blood flow any longer than 20 minutes Perfusion is restored before myocardial necrosis occurs Can progress to NSTEMI/STEMI Chest pain that is prolonged or recurrent, chest pain at rest, or angina which is increasingly severe or frequent May experience dyspnea, diaphoresis, and anxiety as the angina worsens ECG: ST-segment depression and T wave inversion during pain that resolves when pain is relieved TX: oxygen, aspirin, nitrates and morphine NSTEMI Non-ST-Elevation Myocardial Infarction Aka subendocardial infarcy Myocardial infarction which results from a thrombus which has occluded coronary blood flow for greater than 20 minutes and results in myocardial necrosis in the myocardium directly below the endocardium Involves the myocardium directly beneath the endocardium Does not involve the full thickness of the ventricular wall Seen on EKG with ST depression or T wave inversion STEMI ST-Elevation Myocardial Infarction Aka transmural infarct Myocardial infarction which results from a thrombus permanently lodging in the vessel which results in the progression of the myocardial infarction to include the entire thickness of the ventricular wall Involves the myocardium all the way from the endocardium to the epicardium Marked by ST elevation on an EKG I. Evaluate the differences in the extent of an infarction of the myocardium secondary to an NSTEMI and a STEMI and describe the impact this knowledge has on your practice as a nurse practitioner. Type of infarct Extent/severity of infarct Clinical Implications NSTEMI Involves the myocardium directly beneath the endocardium Does not involve the full thickness of the ventricular wall STEMI Progression of the myocardial infarction to include the entire thickness of the ventricular wall Involves the myocardium all the way from the endocardium to the epicardium Highest risk for serious complications and require immediate intervention J. Complications of MI 1. Reperfusion injury can be caused when blood flow is restored: This triggers the release of toxic oxygen radicals, calcium influx, and pH changes that cause persistently open mitochondrial permeability transition pores and contributes to cellular death 2. Myocardial stunning: a temporary loss of contractile function that persists for hours to days after perfusion has been restored, leads to decreased contraction and conduction and can contribute to heart failure, shock, and dysrhythmias 3. Myocardial remodeling: leading to hypertrophy, scarring, and loss of contractile function in areas of the heart distant from the site of infarction 4. Cardiac dysrhythmia are an important cause of pre-hospital death a. PVCs are the most common b. Most common cause of death is V-fib and is frequently associated with cardiogenic shock 5. Functional impairment: a. Decreased cardiac contractility with abnormal wall motion b. Altered left ventricular compliance c. Decreased stroke volume d. Decreased ejection fraction e. Increased LVEDP f. SA or AV node malfunction 6. Left ventricular failure: pulmonary edema/congestion, reduced contractility, and abnormal heart wall motion, usually occur within the first 24 hours 7. Cardiogenic shock results from a large infarct and has a high mortality rate: CO insufficient to maintain normal arterial pressure and to perfuse kidneys and other organs adequately 8. Rupture: a. Ventricular free wall rupture: results in cardiac tamponade from hemorrhage into the pericardial space, occurring in 4-7 days and is associated most commonly with thrombosis of the LAD coronary artery b. Papillary muscle rupture, which causes severe mitral valve regurgitation, is most commonly associated with inferior MIs due to thrombosis of RCA. Present with acute onset of mitral valve regurgitation and L heart failure c. Ventricular septum rupture, which causes a VSD, usually associated with thrombosis in the LAD coronary artery. Produces a L to R shunt, causing R heart failure d. Ruptures associated with audible, harsh cardiac murmur, increased LVEDP, and decreased systemic blood pressure 9. Mural thrombus is a thrombus formed on the endocardium overlying the infarct. Occurs in 10% of MIs and most commonly associated with LAS coronary thrombosis danger of peripheral embolus 10. Ventricular aneurysms: occurs usually 4-8 weeks after STEMI and starts to develop 48 hours after STEMI; bulge results in impaired pump function 11. Post infarction fibrinous pericarditis: a. Person will develop a friction rub 1-3 days post MI b. May develop fever, pain, friction rub, pleural effusion, and arthralgias 12. Thromboembolism: endocardium or pulmonary emboli 13. Sudden death: Unexplained death which results from cardiac cause in persons without symptoms of heart disease or within one hour after the onset of death a. Risk factors: i. Ischemic heart disease, obesity, hyperlipidemia, HTN, LVH, glucose intolerance, NSTEMI ii. Typically occurs between 8am to 11am or 4pm to 7pm b. Etiology: i. Cardiomyopathy, AV stenosis, mitral valve prolapse, cocaine, myocarditis, conduction defects such as Wolff-Parkinson-White syndrome ii. In children, usually attributed to AV stenosis, hypertrophic cardiomyopathy, and Wolff-Parkinson-White Syndrome c. Death typically from a lethal arrhythmia which is usually V-tach d. Dependent of several factors including: degree of L ventricular dysfunction, degree of ventricular ischemia, potential for dysrhythmias, and the individuals age K. Differentiate between the clinical manifestations of a right sided heart failure and a left sided heart failure. L. Analyze the patterns of myocardial injury observed on EKG and describe the implications for treatment of patients experiencing these types of EKG changes. EKG Change Type of injury T wave inversion Myocardial ischemia, blood supply is decreased ST Segment Elevation Myocardial injury Elevation greater than 1mm above baseline or ST depression Injury denotes an acute infarct ST Depression Indicates subendocardial infarction and represents a small area affected Not transmural like ST elevation is Q waves Myocardial infarction Q waves greater than 25% of the QRS complex height or more than 0.04 sec wide (1 small box) Pathologic Q waves may indicate necrosis, dead cells which cannot depolarize Q waves may be old and indicated an old MI: characteristic post-STEMI, developing some hours later Presence of Q waves with ST elevation indicates acute infarct M. EKG and Location of MI 1. Anterior wall MI (LAD): ST elevation or Q waves, or ST depression in V1-V4 2. Anteroseptal MI (LAD): ST elevations, Q waves or ST depression in V4-V6 3. Anterolateral MI (LAD or LCX): ST elevations, Q waves or ST depression in V4-V6 4. Lateral Wall MI (LCX): ST Elevations, Q waves or ST depression in I, aVL 5. Inferior Wall MI (RCA): ST Elevations, Q waves or ST depression in II, III, aVF Valvular Heart Disease I. Examine the risk factors, etiology, clinical manifestations of disorders of the heart valves. A. Analyze the etiology, clinical manifestations, and pathophysiology of valvular heart disorders which affect the aortic and mitral valves and describe the implications this has on the care you provide as a nurse practitioner. Valvular Heart Disease Etiology Clinical Manifestations Pathophysiology Aortic Stenosis Most common valvular disease in adults Most common: secondary to aortic valve calcification in persons 60 years of age Forward flow (out of the aorta) may be decreased and can result in syncope or chest pain Reduced systolic BP and Normally the valve measure 3cm2 Clinical manifestations develop when the valve is <1cm and said to be severe when <0.5cm2 Narrowed valve prevents the outflow and older Congenital: occurs in persons <30 years old, bicuspid valve Inflammatory damage from rheumatic heart disease narrowed pulse pressure Murmur heard at the second intercostal space and may radiate to the neck of the left ventricle resulting in L ventricular hypertrophy, which if not treated leads to increased pressure and volume in the L atrium and causes pulmonary HTN and edema Hypertrophy increased myocardial O2 demand that coronary arteries may not be able to supply, ischemia occurs, attacks of angina, dysrhythmias, myocardial infarction, and heart failure Mitral Stenosis Most commonly caused by rheumatic fever More common in women Dyspnea, hemoptysis, atrial fibrillation, dysphagia, and pulmonary hypertension Diastolic murmur, accentuated S1 (opening snap) Characterized by a narrowing of the mitral valve to <2.5 cm2 (normally 4- 6) Results in an increased volume and pressure in the L atrium, which results in atrial hypertrophy and can ultimately increase the pressure and volume in the pulmonary circulation leading to pulmonary edema and R ventricular failure Aortic Regurgitation Most commonly caused by aortic root dilation Infective endocarditis, rheumatic fever, aortic dissection, CoarC, aortitis from syphilis or ankylosing spondylitis (connective tissue disorder) Marked by an early diastolic murmur radiating to neck Incompetent closure of the aorta results in a back flow of blood into the L ventricle Acutely causes an increase in LVEDP, a decreased in SBP and stroke volume, and normal or decreased pulse pressure, and a decrease in cardiac output Chronic AV: the LVEDP normalizes, L ventricular hypertrophy occurs, SBP is increased and DBP decreases, cardiac output is normal and the pulse pressure is increased; ultimately the volumes and pressure back up into the L atrium and pulmonary circulation Untreated can lead to L ventricular hypertrophy and dilation followed by heart failure Mitral Regurgitation Most common cause is mitral valve prolapse, a type of mitral valve regurgitation, more Dyspnea, rales, pansystolic murmur with S3 and S4 heart sounds Characterized by the incomplete closure of the mitral valve; blood in the L ventricle backs up into the L atrium during ventricular systole common in women Ruptured papillary muscle dysfunction, infective endocarditis, dilated cardiomyopathy, myocarditis, nonbacterial endocarditis Mitral valve prolapse: sticking type of chest pain Mitral incompetence can be tolerated for years and clinical manifestation are related to heart failure will lead to L atrial dilation and hypertrophy L heart failure increased pulmonary vascular pressure and volume and pulmonary edema In acute mitral regurgitation caused by MI, surgical repair must be done emergently II. Discuss the etiology, clinical manifestations, pathophysiology of endocarditis. A. Infective endocarditis: general term used to describe infection and inflammation of the endocardium especially the cardiac valves B. Etiology: 1. Bacteria: most common cause especially streptococci, staphylococci, and enterococci 2. Viruses, fungi, rickettsia, and parasites 3. Risk factors: trauma, congenital heart disease, Valvular heart disease, and the presence of prosthetic valves C. Pathophysiology: Requires three key elements: 1. Endocardial damage: a. See risk factors b. Turbulent flow effects atrial surface of valves leading to damage that exposes the basement membrane causing a inflammatory reaction 2. Blood-borne microorganism adherence to the damaged endothelial surface using adhesins 3. Formation of infective endocardial vegetations: a. Bacteria infiltrate the sterile thrombi and accelerate fibrin formation by activating the clotting cascade b. Vegetative lesions usually form on heart valves and surrounding tissues: are embedded in protective fibrin clots and are inaccessible to host defenses c. Embolization from vegetations can lead to characteristic skin changes: petechiae, splinter hemorrhage, Osler nodes, and Janeway lesions D. Clinical Manifestations: 1. Can be acute, subacute, or chronic 2. Classic findings: fever, cardiac murmur, petechial lesions of the skin, conjunctiva, and oral mucosa 3. Osler nodes: painful erythematous nodules on the pads of the fingers and toes 4. Janeway lesions: non-painful hemorrhagic lesions on the palms and soles 5. Weight loss, back pain, night sweats, and heart failure 6. CNS: splenic, renal, and pulmonary peripheral arterial, coronary, and ocular emboli may lead to a wide variety S/S Heart Failure I. Evaluate the risk factors, etiology, clinical manifestations and pathophysiology of disorders which affect myocardial contraction. A. Heart Failure: pathophysiologic condition in which the heart is unable to generate an adequate cardiac output such that there is inadequate perfusion of tissues or increased diastolic filling pressure of tissues or increased diastolic filling of the left ventricle or both 1. A syndrome which results from myocardial injury 2. It is the end results of myocardial damage from ischemia heart disease, cardiomyopathies, valvular heart disease, myocarditis, and results in a constellation of clinical manifestations which are consistent with volume overload, poor perfusion secondary to pump failure 3. Risk factors include age, ischemic heart disease, obesity, diabetes, HTN, excessive ETOH use, CHD, valvular heart disease, myocarditis, cardiomyopathies, and renal failure 4. High-output failure: a. Inability of the heart to adequately supply the body with blood-borne nutrients despite adequate blood volume and normal or elevated contractility b. Common causes: anemia, septicemia, hyperthyroidism, and beriberi (thiamine deficiency) B. Analyze the etiology, risk factors, clinical manifestations, and pathophysiology of systolic and diastolic heart failure and describe the clinical implications for risk reduction, patient education, and treatment. Type of Heart Failure Etiology Clinical Manifestations Pathophysiology Systolic HF HF with Reduced Ejection Fraction (HFrEF) MI: most common Myocarditis and cardiomyopathi es Hypertension- hypertrophic cardiomyopathy : increased PVRincrease d afterload increased L ventricle workload hypertrophy Pulmonary edema secondary to the back up of blood and high pressures into the pulmonary circulation Edema from the sodium and water retention Dyspnea, orthopnea, cough of frothy sputum, fatigue, decreased urine output, edema BNP: used to diagnose HF and give insight to severity Beta blocker are given to patients with HErEF to block the effects of RAAS: medications will prevent the progression of the heart failure, remodeling and may improve the systolic function and decrease mortality Heart failure with reduced ejection fraction (HFrEF) Most commonly refers to the L side of the heart but R ventricular systolic dysfunction can happen too It is impairment in the L ventricular contraction (systole) Decrease in contractility in the L ventricle will lead to a decrease in stroke volume, decrease in cardiac output, and increase in L ventricular end diastolic volume (preload) This is the first step of ventricular remodeling, which over time, as preload increased causes a dilation of the ventricle and further compromises contraction and cardiac output Ventricular remodeling leads to further pathological deterioration of the myocytes the process is initially triggered by some pathologic insult to the myocardium which causes damage that leads to the dysfunction described above Myocardial systolic dysfunction, low cardiac output, results in two different processes being triggered (1) Baroreceptor activation: located in the L ventricle, aortic arch, and carotid sinus, detect the low CO and low BP and notify the medulla which stimulated the SNS the SNS stimulated the release of catecholamines epinephrine and norepinephrine which cause vasoconstriction and leads to increased afterload, increased BP and increased HR this increases the work load of the heart and causes hypertrophy and dilation of the L ventricle and further impairs contractility (2) Renin Angiotensin Aldosterone System: activated by decreased renal blood flow, end result of the RAAS is the release of angiotensin II and aldosterone AGII causes vasoconstriction which increases afterload and contributes to L ventricular hypertrophy, dilation, and worsening contraction; aldosterone enhances renal sodium retention and thereby water, increasing afterload again and contributing to L ventricular hypertrophy, dilation, and worsening contraction Heart muscle exhibits progressive changes in myocyte myofilaments, decreased contractility, myocyte apoptosis and necrosis, abnormal fibrin deposition in the ventricle wall, myocardial hypertrophy, and changes in ventricular chamber geometry Hypertrophy mediated by catecholamines and angiotensin II Diastolic HF More common in women Most commonly caused by hypertension- induced- hypertrophy or Dyspnea on exertion and fatigue Pulmonary edema Pulmonary HTN and R HF Characterized by the presence of pulmonary congestion in the setting on normal left systolic EF, stroke volume, and cardiac output Pathologically results from the inability of the myocytes to actively pump calcium from the cytosol which impairs myocardial ischemia which results in remodeling Inotropes not indicated in tx because ejection fraction and contractility are not affected ventricular relaxation L side: Since the L ventricle is unable to completely relax, it does not fill with as much blood, the contraction of the heart is completely normal and what blood the ventricle has, it is able to pump forward effectively because of the defect in relaxation the filling of the ventricle results in increased LVEDP the pressure backs up into the left atrium and the pulmonary circulation and results in pulmonary edema over time pulmonary HTN and R side HF Also results from sustained activation of RAAS and the SNS C. Explain the process of ventricular remodeling and describe the implications for clinical practice. D. Differentiate between the etiology, risk factors, clinical manifestations and pathophysiology of left- sided and right-sided heart failure. Type of Heart Failure Etiology Clinical Manifestations Pathophysiology Left-Sided HF Synonymous with HFrEF Congestive heart failure Right-Sided HF Most common cause if L-Side HF Without L-sided HF: causes include -Primary lung disorders such as COPD, cystic fibrosis, ARDS Jugular vein distention, hepatosplenomegaly, peripheral edema Failing R ventricle which results in its inability to pump blood forward to the pulmonary circulation When pressures are too high in the L ventricle, the back flow of blood into the pulmonary circulation causes higher pressures in the pulmonary circulation against which the R ventricle must pump -Right ventricular MI, cardiomyopathies, or pulmonic valve disease R ventricle is unable to effectively pump against the increase in pressure and ultimately dilates and fails As a result, the pressure and volume backing up causes right atrial hypertrophy, jugular vein distention, hepatosplenomegaly, peripheral edema Examine the pathological basis of congenital heart defects. Congenital Heart Defects II. Explain fetal circulation A. Placenta is the organ in which oxygen exchange occurs and that removes waste products and provides nutrients. Without it the fetus would not remain viable. B. During fetal growth and development blood flows is different that it is after birth. 1. The umbilical vein receives oxygenated blood from the placenta 2. The umbilical vein connects to the hepatic circulation but also connects to the inferior vena cava by the ductus venosus. 3. The ductus venosus allows the oxygen-rich blood to enter the inferior vena cava and some blood does enter the hepatic circulation. 4. From the inferior vena cava, the blood is emptied into the right atrium. 5. The most oxygenated blood in the right atrium is shunted through the foramen ovale (this is an opening between the right and left atria), into the left atria. 6. The blood then enters the left ventricle and is pumped out to the head and the rest of the body. 7. The deoxygenated blood also enters the right atrium just as the oxygenated blood does. 8. There are two streams that help to keep the blood separate. a. Sixty percent of the blood in the right atrium (which is oxygenated blood) will be moved forward as described above. b. The remaining 40% of the blood is mixed blood (oxygenated and deoxygenated) and will move from the right atrium, to the right ventricle, and into the pulmonary artery. 9. From the pulmonary artery it will pass through the patent ductus arteriosus (which is a connection between the pulmonary artery and the aorta) into the aorta. 10. The aorta will connect with the umbilical artery, where the blood will go back to the placenta to exchange gas, get rid of waste products and pick up nutrients. 11. The right side of the heart has the higher pressure prior to birth. After birth this changes with the neonate’s first breath and the left side of the heart becomes the one with the higher pressure III. CHD are the most common heart diseases which affect children A. Preterm babies more commonly affected than full term babies B. Etiology is unknown in most cases (90%) C. Known causes 1. Genetic environmental interactions: multifactorial factors 2. Primary genetic factors: single gene disorders, chromosome disordered 3. Sole environmental factors: isotretinoin, alcohol, maternal rubella infection D. Maternal risk factors : 1. Age >45 2. Prior child born with CHD 3. Poor controlled diabetes during pregnancy 4. Alcohol use during pregnancy 5. Congenital infection during pregnancy (rubella) 6. Aspirin intake 7. Systemic lupus 8. Diphenylhydantoin (phenytoin) intake IV. Evaluate the risk factors, etiology, clinical manifestations, and pathophysiology of patent ductus arteriosus, atrial septal defect, ventricular septal defect and describe the implications this has for the diagnosis and medical management of patients with these three congenital heart defects. Disease Etiology Clinical Manifestations Pathophysiology Patent Ductus Arteriosus Associated with rubella, respiratory Machine-like murmur hear continuously through systole Results from a PDA which does distress syndrome after birth, complete transposition of the great vessels and tetralogy of fallot Increased incidence in premature infants and diastole Large PDA: S/S of pulmonary overcirculation not close after birth Shunt left to right that leads to increased pulmonary flow, increased pulmonary return to the LA and LV with increased workload on the L side of the heart and potentially increased R side pressures Reversal of the shunt may occur if pulmonary hypertension develops from increased blood flow through the pulmonary artery deoxygenated blood will enter the aorta below the level of the subclavian artery cyanosis in the lower body but normal in the upper body Atrial Septal Defect Most common CHD in adults Most common cause is a PFO that does not close Associated with fetal alcohol syndrome and Down syndrome Soft midsystolic murmur at the upper sternal border Generally asymptomatic and rarely display symptoms pulmonary overciruclation Increased risk for developing an embolus if unrepaired (1) Ostium primum defect: low in septum, may be associated with AV valve abnormalities (2) ostium secundum: center of the septum (3) sinus venosus defect: high in atrium near superior vena cava and RA junction Moderate to large ASDs may increase pulmonary flow ASD closure before school age to prevent pulmonary HTN and R ventricular failure Ventricular Septal Defect Most common CHD Multiple VSDs associated with tetralogy of fallot and other congenital disorders such as cri du chat, fetal alcohol syndrome, Also associated with the development of ASD, PDA, Coarc, aortic valve stenosis Harsh holosystolic murmur at the lower, left sternal border Increased risk for endocarditis Large VSD: S/S HF, poor weight gain Infants with symptoms of HF and poor weight gain should have their VSD corrected ASAP PA band placement Results in a communication (a hole) in the interventricular septum Allows blood to move from LR Eisenmenger syndrome: LV, LA, and PA hypertrophy, pulmonary overcirculation increased pulmonary vacular resistance exceeding systemic vascular resistance Right to left shunting deoxygenated blood Contraindications for closure: Eisenmenger syndrome or evidence of pulmonary vascular obstructive disease flows into systemic circulation and cyanosis occurs A. Differentiate between the pathophysiology of right to left and left to right heart shunts. 1. Left to Right a. Shunt oxygenated blood from the left side of the heart mixes with the right side of the heart b. The SaO2 on the left side of the heart is 95% and normally on the right side 75% c. As the blood mixes, the SaO2 on the right side of the heart is increased to 80% or more d. Pathological consequence are directly caused by the amount of blood being shunted e. Volume overload occurs in the R side of the heart leading to: i. Pulmonary hypertension ii. R ventricular hypertrophy: secondary to pulmonary HTN iii. Left ventricular hypertrophy: secondary to increased blood return to the LV iv. Eisenmenger: later reversal of the shunt (left to right) because the right side of the heart will end up with higher pressures than the left v. Considered late onset cyanosis and will result in clubbing of the fingers 2. Right to Left a. Deoxygenated blood is mixed with oxygenated blood b. SaO2 on the L side of the heart drops from 95% to 80% or lower c. Development of cyanosis is dependent on how low the SaO2 is on the L side of the hear d. If SaO2 remains 85% or higher, cyanosis is not likely to develop because the shunting is minimal e. An SaO2 below 80% is indicative of a higher amount of blood being shunted and will result in cyanosis f. Tetralogy of Fallot is the most common cyanotic CHD g. Characteristic of defects that decrease pulmonary blood flow and mixing lesions [Show More]
Last updated: 2 years ago
Preview 1 out of 26 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$15.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jul 30, 2021
Number of pages
26
Written in
Additional information
This document has been written for:
Uploaded
Jul 30, 2021
Downloads
0
Views
60