Chemistry > QUESTIONS & ANSWERS > North Carolina State UniversityCH 202 Lab 11 PostLab - Chemical Kinetics. Current Score : 23.64 / 25 (All)
North Carolina State UniversityCH 202 Lab 11 PostLab - Chemical Kinetics. Current Score : 23.64 / 25. LATEST FOR 2021/2022
Document Content and Description Below
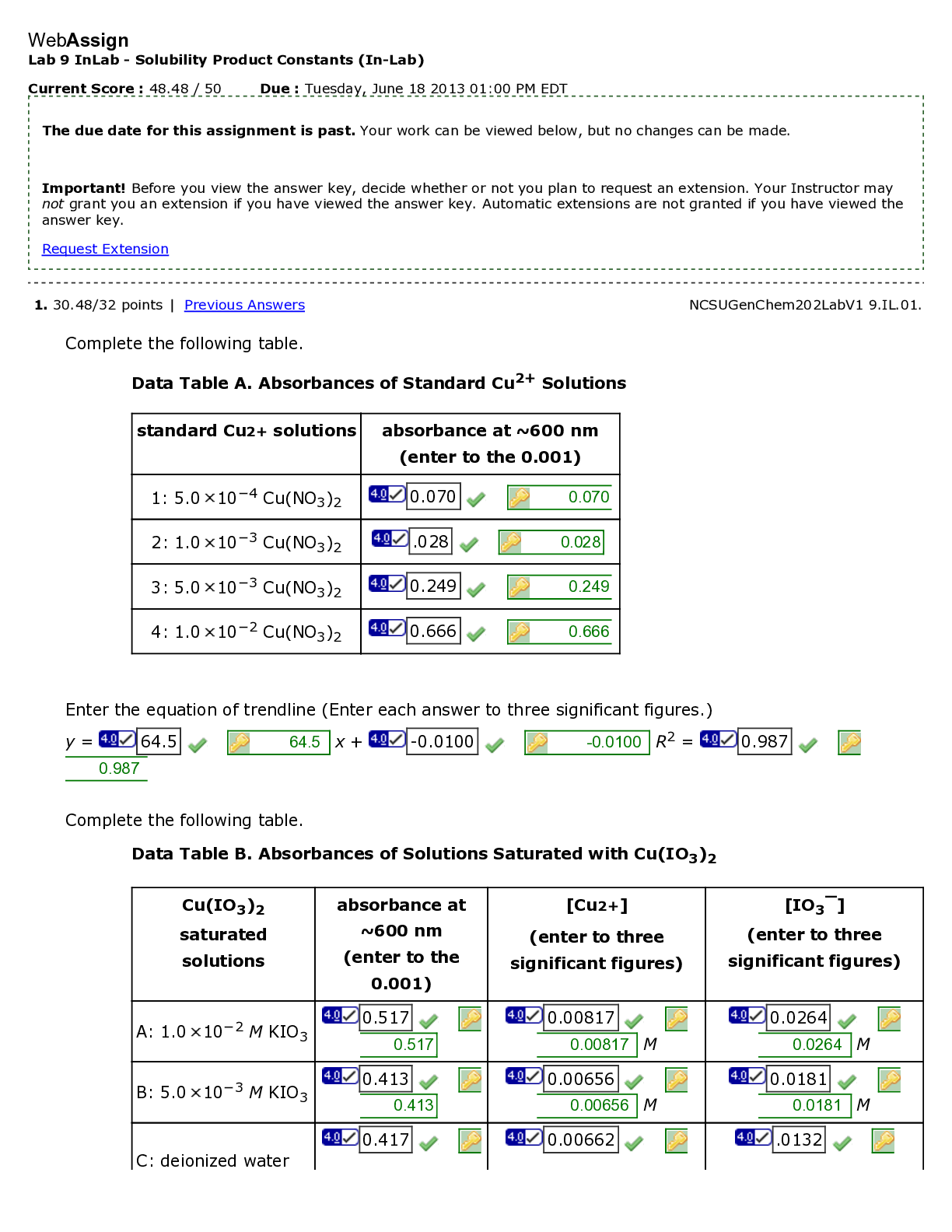
Current Score : 23.64 / 25 Due : Saturday, June 22 2013 11:00 PM EDT 1. 13.64/15 points | Previous Answers NCSUGenChem202LabV1 11.POST.01. Refer to Data Table B, in which you entered rate constants. I... n the spaces below, enter your rate constants, in M-1s-1. Do not include units. What is the range of these values from highest to lowest? 0.0024 0.0024 What is the average of these values? 0.0064 0.0064 The remainder of this question is designed to help you decide if the data from one of your experiments can be discarded. To quantitatively "throw out" a measurement, you must perform a statistical test on your data called a Q-Test. The formula for the Q-test is Qcalculated = gap/range where gap = (questionable value ! next closest number) and range = (largest number ! smallest number). Qcalculated is then compared to Qtable. If Qcalculated > Qtable then the questionable datum can be discarded. Q (90% confidence) number of measurements 0.76 4 0.64 5 Lab 11 PostLab - Chemical Kinetics (Postlab) WebAssign The due date for this assignment is past. Your work can be viewed below, but no changes can be made. Important! Before you view the answer key, decide whether or not you plan to request an extension. Your Instructor may not grant you an extension if you have viewed the answer key. Automatic extensions are not granted if you have viewed the answer key. Request Extension 0.56 6 What is the gap of these values (questionable value ! next closest value)? (Take the absolute value if necessary to make this number positive.) -0.08 0.001 What is Qcalculated for your data above? 0.40 0.42 Which Qtable will you compare your Qcalculated to? Can the questionable value be discarded based on your Q-test results? Additional Materials Chemical Kinetics 2. 9/9 points | Previous Answers NCSUGenChem202LabV1 11.POST.02. In your lab write-up, three possibilities for the mechanism of the rate determining step were listed. 1. The rate-determining step has two iodide ions coming together. 2. The rate-determining step involves a persulfate ion decomposing. 3. The rate-determining step has an iodide ion and a persulfate ion coming together. Which mechanism did your experiment confirm? the third the third Now consider what would have happened if one of the other mechanisms had been correct. The initial reaction is run with arbitrarily selected concentrations of reactants. Then, concentrations are varied systematically. Consider the following possibilities and select the correct outcome for each experiment. (a) If the first mechanism is correct, what should happen to the rate if the concentration of iodide ion is doubled and other concentrations are held constant? (b) If the first mechanism is correct, what should happen to the rate if the concentration of persulfate ion is doubled and other concentrations are held constant? (c) If the second mechanism is correct, what should happen to the rate if the concentration of iodide ion is doubled and other concentrations are held constant? (d) If the second mechanism is correct, what should happen to the rate if the concentration of persulfate ion is doubled and other concentrations are held constant? Additional Materials Chemical Kinetics The rate will not change. The rate will increase by a factor of four. The rate will decrease by half. The rate will double. The rate will increase by a factor of four. The rate will decrease by half. The rate will double. The rate will not change. The rate will increase by a factor of four. The rate will not change. The rate will decrease by half. The rate will double. The rate will not change. The rate will decrease by half. The rate will double. The rate will increase by a factor of four. 3. 1/1 points | Previous Answers NCSUGenChem202LabV1 11.POST.03. You are now answering the last question of the last Postlab assignment in CH202. Which of the following words or phrases best describes your emotions at this point? Additional Materials Chemical Kinetics YEE-HAW!!! I am SO outta here! Glory Hallelujah! Aw, nuts. All good things must end. I can't wait for my next chemistry lab course [Show More]
Last updated: 2 years ago
Preview 1 out of 4 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jul 31, 2021
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Jul 31, 2021
Downloads
0
Views
80