Chemistry > STUDY GUIDE > CATIONIC REARRANGEMENT University of Calgary CHEM 453 (All)
CATIONIC REARRANGEMENT University of Calgary CHEM 453
Document Content and Description Below
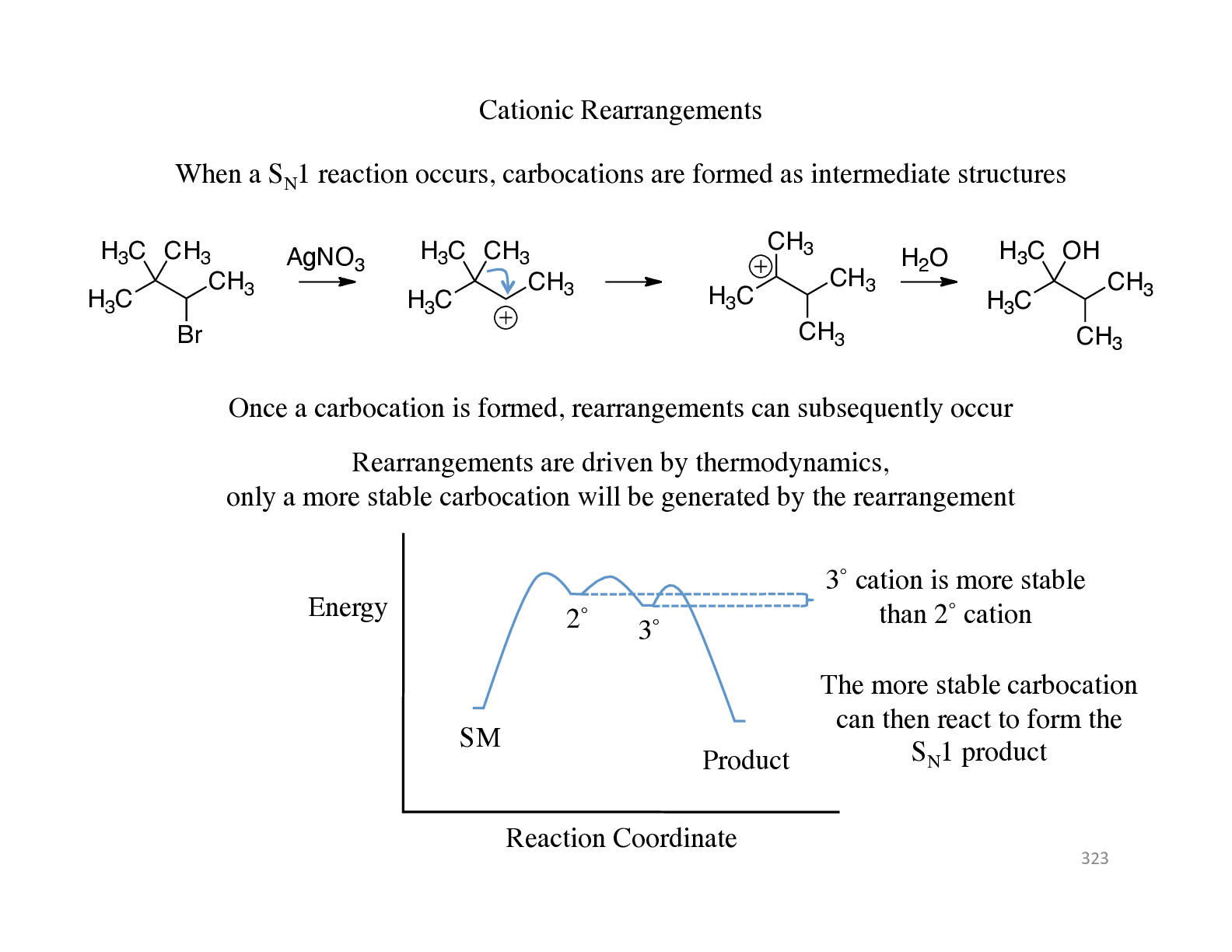
Cationic Rearrangements When a SN1 reaction occurs, carbocations are formed as intermediate structures H3C H3C CH3 CH3 Br AgNO3 H3C H3C CH3 CH3 Reaction Coordinate Energy SM 2˚ 3˚ Prod... uct 3˚ cation is more stable than 2˚ cation H3C CH3 CH3 CH3 H2O H3C H3C OH CH3 CH3 Once a carbocation is formed, rearrangements can subsequently occur Rearrangements are driven by thermodynamics, only a more stable carbocation will be generated by the rearrangement The more stable carbocation can then react to form the SN1 product 323# Cationic Rearrangements Rearrangements are known to occur by migrating a hydrogen, alkyl groups, or aryl groups (when an alkyl or aryl group migrates, it is called a Wagner-Meerwein shift) A 1,2 shift is the most common type of rearrangement In order to allow a rearrangement to occur, the bonding orbital of the migrating group needs to be coplanar with the carbocation orbital H H H H3C H3C H H H H3C CH3 Newman projection Only in this conformer is hydrogen aligned with empty p orbital H H H3C H3C H The orbital of the C-H bond must be aligned with the empty p orbital to allow a rearrangement to occur 324# Cationic Rearrangements Rearrangements only occur with cations, not with anions or radicals Process occurs with an orbital on an adjacent atom interacting with the empty p orbital of the carbocation H3C H H CH3 CH3 CH3 CH3 H H3C H CH3 CH3 H H3C H Consider the orbital interactions for the transition state for this process Bonding molecular orbital Antibonding molecular orbital In a cation rearrangement, 2 electrons are involved in a bonding molecular orbital In a radical or anion rearrangement, additional electrons would be placed in antibonding molecular orbitals (therefore a less stable process) 325# Cationic Rearrangements Carbocations are generally unstable species so therefore they are intermediate structures, not final products After generation and possible rearrangements, the carbocations must react to form a stable product H3C H3C CH3 CH3 Br AgNO3 H3C H3C CH3 CH3 H3C CH3 CH3 H3C H H2O H3C CH3 CH3 CH3 Two of the common pathways for the carbocation to react is either reacting with a base to form an E1 product H3C H3C OH CH3 CH3 H2O Or to react with a nucleophile to form an SN1 product SN1 E1 326# Cationic Rearrangements A third possibility to form products in carbocation reactions is to form a strong bond after the formation of the carbocation This is the energetic driving force in pinacol rearrangements HO OH H3C CH3 H3C CH3 Pinacol H+ H2O OH H3 [Show More]
Last updated: 2 years ago
Preview 1 out of 15 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 12, 2022
Number of pages
15
Written in
Additional information
This document has been written for:
Uploaded
Dec 12, 2022
Downloads
0
Views
98






.png)

.png)

















