Chemistry > Lab Report > Seaquam SecondaryCHEM IBChemistry IA, Final (All)
Seaquam SecondaryCHEM IBChemistry IA, Final
Document Content and Description Below
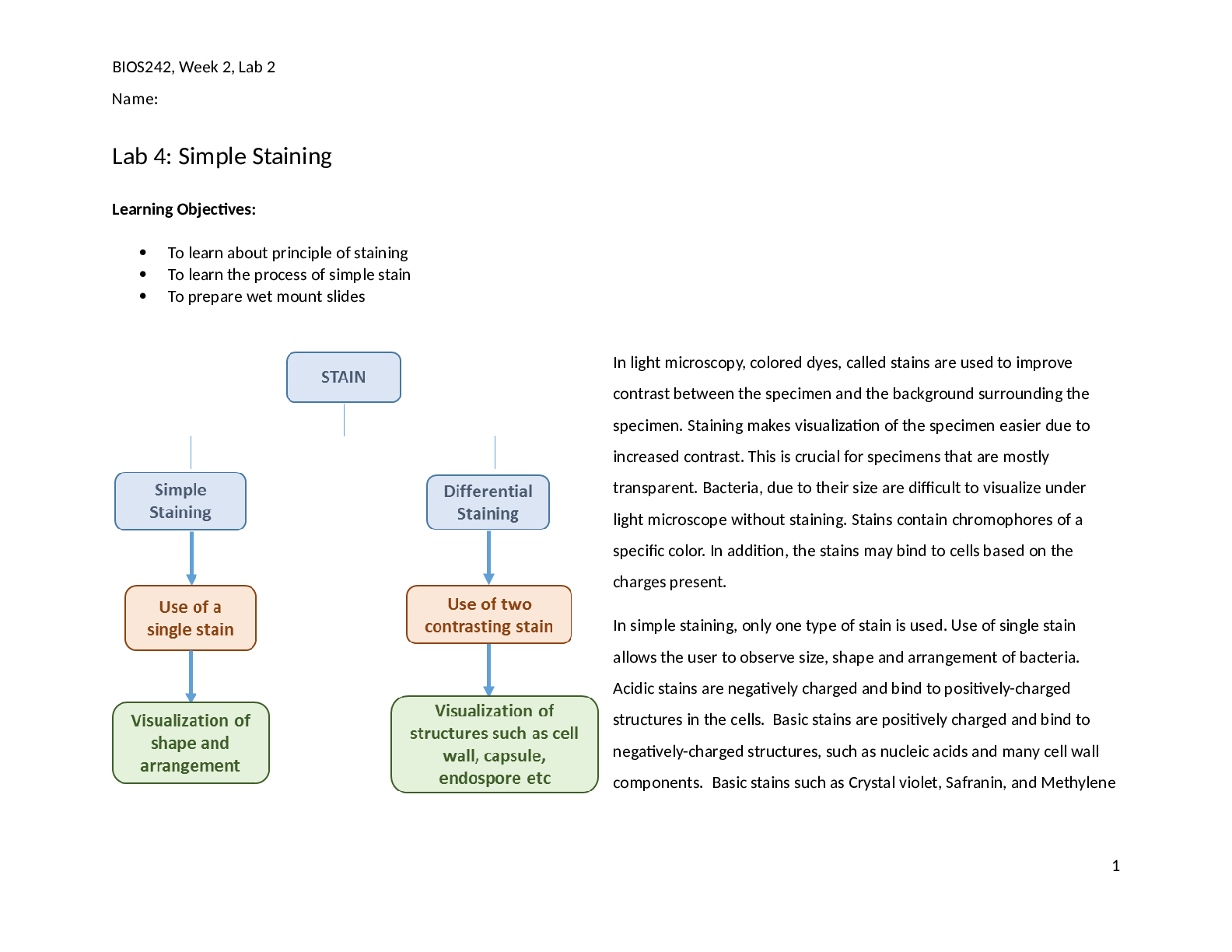
Name: Sarthak Garg Candidate Session Number: 006672-0071 Personal Code: fph319 Exam Session: May 2021 School: Seaquam Secondary School Teacher: Ms. Stephenson Subject: IB Chemistry HLGarg – 00 ... 6672-0071- 2 Investigation of Aspirin Decomposition Introduction Medication has become a human right. It has grown dramatically in the past few years. So much so that it is one of the most easily accessible items in the world. One of the most commonly used drug in the world is Aspirin, created by Felix Hoffmann. It is a platelet aggregation inhibitor. It is used for a lot of medical applications. One of its uses is as an anti-inflammatory drug or to reduce chronic pain etc. [Asp]. Aspirin is chemically known as Acetylsalicylic acid. Its chemical formula is C9H8O4. Since my father is a pharmacist, I have been exposed to biochemistry of medicines from an early age. The decomposition of these medicines have intrigued me for a long time. I am interested in how these medicine are preserved and what their optimal conditions are. Aspirin decomposes because of hydrolysis, reacting with water. It decomposes in the following manner: H2O + C9H8O4 ---> C7H6O3 + CH3COOH Figure 1 Acetylsalicylic acid (Aspirin) decomposition The products formed are acetic acid and salicylic acid [Car88]. For this investigation, the main compound formed is Salicylic acid. This compound is what further reacts to show rate of decomposition. This compound reacts with Iron (III) nitrate in the solution to form a conjugate, Ferric Salicylate, with a violet color [Nag75] as following: Iron (III) Nitrate + Salicylic Acid → Ferric Salicylate + Nitric Acid Fe(NO3)3 + 3CH4(OH)COOH Fe(C → 6H4(OH)COO) 3 + 3HNO3 Figure 2 Ferric Salicylate This violet color increases the absorption of the solution. This change in absorption is measured with a colorimeter. This is then compared with the calibration curve which is prepared separately. The question arises for the different factors that affect the rate of decomposition of aspirin. Since optimal conditions are required for a medicine to preserve it and to maintain its effectiveness, pH may form a very important factor that must be taken into account. This investigation will allow me to determine, or to guide me on whether the pH really forms an important factor in terms of aspirin decomposition. Thus the research question: To what extent does the pH of the solution affect the rate of decomposition of Acetylsalicylic Acid in ethanol?Garg – 006672-0071- 3 Aim: To investigate the effect of pH of the solution on decomposition of Acetylsalicylic Acid, aspirin, in ethanol. Experimental Hypothesis (H1): When the pH of the solution in which the aspirin disassociates is decreased, that is, the concentration of protons in the solution is increased, the absorbance change rate will decrease and when the pH is increased, the absorbance change rate will go up as according to le Chatelier's principle [Kno85], when the concentration of proton’s is increased, the reaction would shift towards the side with less protons which the side with a lower concentration of protons to reach equilibrium. The side with less protons in this case is the reactant side, the side with aspirin because aspirin is a weaker acid than salicylic acid which means that it will disassociate less and so there will be more protons on the product side, whereas increase in pH would cause the reaction to shift right to increase the protons. This will impact the rate of decomposition and thus the absorbance change rate. Null Hypothesis (H0): There will be no change in the absorbance change rate or any change will be due to random chance. Safety Do not ingest any materials from the experiment. Always wear gloves when handling any lab materials. Carefully dispose of all the materials as instructed. Clean work space and all equipment. Ethics Discard of materials down the drain can cause environmental damage. If ingested, the tablets may cause damage to the researcher. Materials Chemicals: o Ethanol o Salicylic Acid, 50g o PH Buffers: 8.5, 7.0, 6.0 o Aspirin Tablets (Bayer 325mg) o Fe(NO3)3 · 10H2O Glassware: o 5 100ml volumetric flask o 5 50ml beakers o 100ml beakers o Glass Stirring rod o 1ml Dropper Equipment o Magnetic stirrer o Colorimeter o Lab Quest Independent Variable: The pH buffers in which the solution is made.Garg – 006672-0071- 4 o 7.0, 8.0 and 6.0 Dependant Variable: The absorbance of the acetylsalicylic acid – ethanol solution. Confounding variables and Controls Confounding Variables Why control is needed: How to control: Time between measuring absorbance for trials of same condition at the same time period. If the time between measurements of different trials is not kept constant, different time will be allowed for aspirin to decompose and so measurements will differ and collude the results Measure the trials within 10 seconds of each other every time. Surrounding temperature A difference in temperature between trials or conditions could cause temperature to affect the rate of decomposition as temperature can increase reaction rate. Perform all the trials with all the conditions at approximately the same time and weather to maintain surrounding temperature. Wavelength of light used to measure absorbance Different wavelength of light used can cause different absorbance value just because they are absorbed differently by the molecules. This can produce false data due to wrong absorption. Use the same wavelength of light for all of the conditions: 565nm. Procedure Preparing Calibration Curve Solution A: 1. In a 50ml beaker, add 20 ml of buffer 7.0 solution and 10ml of ethanol. 2. Dissolve 0.100g of salicylic acid in the solution. 3. Stir with a glass stirring rod till no precipitate is visible. 4. Using a funnel, transfer this solution in a 100ml volumetric flask. 5. Dilute the solution to 100ml mark with 7.0 buffer solution. 6. Stir for 2 minutes with a magnetic stirrer. 7. Preparing ethanol-buffer solution: a. In a 50ml beaker, mix 15ml of ethanol with 15ml of 7.0 buffer solution. 8. Take 2.5ml of salicylic acid solution and dilute it to 25 ml in a 25ml volumetric flask with the ethanolbuffer solution from step 7. Stir with a magnetic stirrer for 2 minutes. This is Solution A. Preparing Solution B: 1. In a 50ml beaker, pour 20ml of 7.0 buffer solution. 2. Add 1.00 g of Fe (NO3)3·10 H2O in the solution. 3. Stir with a magnetic stirrer till no precipitate is visible. 4. Transfer the solution in a 25 ml volumetric flask and dilute the solution to 25ml with 7.0 buffer solution. This is Solution B. [Show More]
Last updated: 3 years ago
Preview 1 out of 15 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$15.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 25, 2021
Number of pages
15
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 25, 2021
Downloads
0
Views
170

.png)


.png)









.png)



