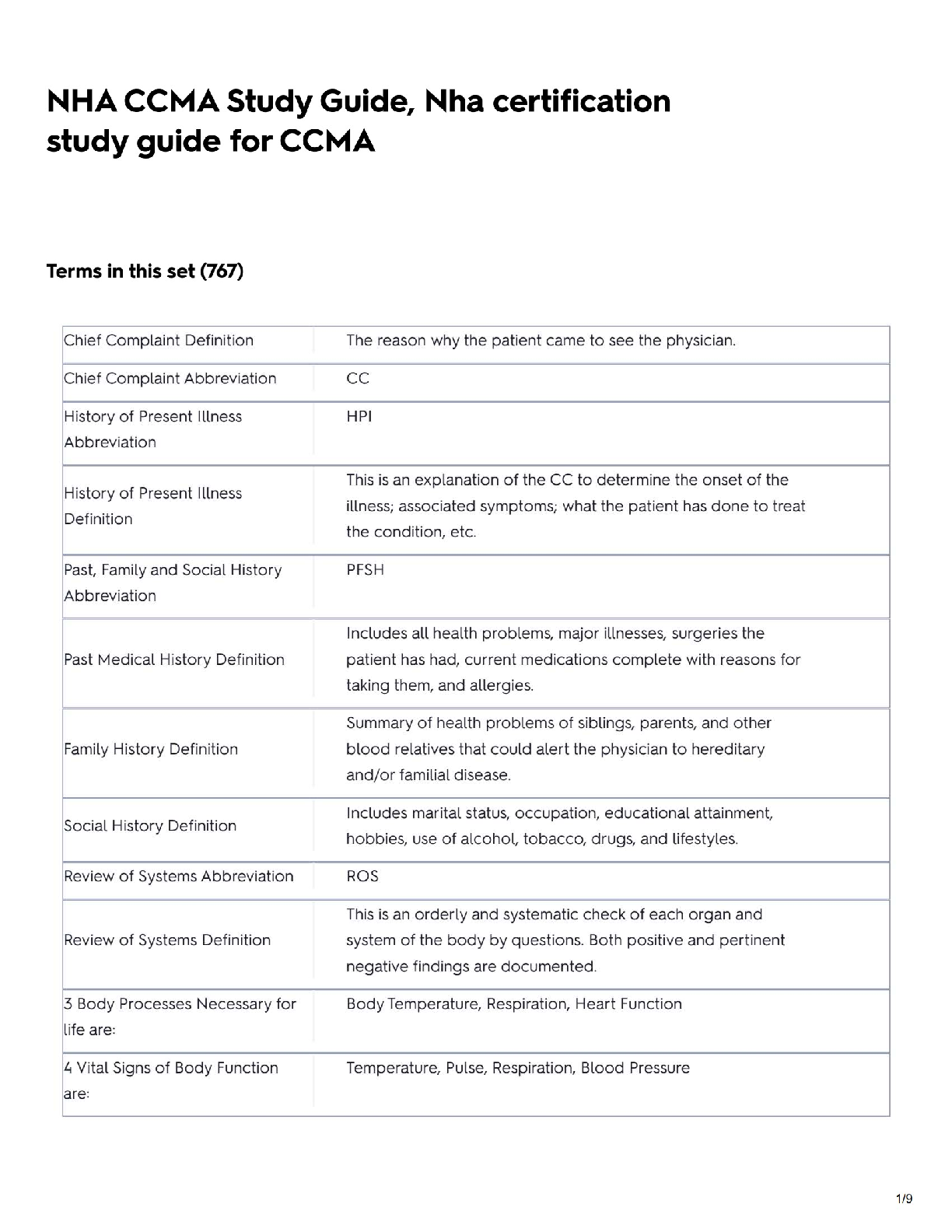
CHM 113 _07_Enthalpy_PostLab-1
Document Content and Description Below
CHM 113 _07_Enthalpy_PostLab-1
Enthalpy and Specific Heat Roberto Flores
10/07/2019
TOTAL: 20 points
CHM 113 POST-LAB
Enthalpy and Specific Heat
1. Insert ONE picture of yourself in f
...
ull PPE here (include the packet of hand warmer).
**Remember to (1) show your full body so that we see you are wearing shoes; (2) wear your safety glasses, lab coat, and gloves; (3) cover your lower legs (socks are not optional, even in Arizona); (4) tie back long hair in a ponytail or a bun; (5) remove jewelry.
2. Complete the following table with your experimental data for the masses of the water and the unknown metal strip in Part 1.
Table 1: Mass Data
Mass (g)
Trial 1 Trial 2 Trial 3
Water 45.8g 47.7g 46.5g
Metal Strip 20.7g 19.3g 20.2g
3. Complete the following table with your experimental data for the temperature changes for the water in your calorimeter in Part 1, and calculate the temperature change (ΔT) for each trial. Show your work below the table and include units.
∆? = ?????? − ????????
Table 2: Specific Heat Data
Time (minutes) Temperature (°C)
Trial 1 Trial 2 Trial 3
Initial 20.7 20.2 19.9
1 minute 21.8 22.1 21.9
2 minutes 21.9 22.2 22.0
3 minutes 22.3 22.2 22.3
4 minutes 22.4 22.5 22.4
5 minutes 22.0 22.5 22.4
ΔT 1.3 2.3 2.5
Calculations of ΔT
Trial 1: 22.0-20.7=1.3
Trial 2: 22.5-20.2=2.3
Trial 3: 22.4-19.9=2.5
4. Calculate the specific heat (C or Cs) of the unknown metal strip for each trial here. What is the average specific heat for all three trials? Show your work and include units.
Hint: Table 2 shows the ΔT for the water. For the ΔT of your metal, consider the initial temperature of the metal (Part 1, Step 9 in Procedure) as well as the final temperature once the calorimeter contents reached equilibrium.
?????? + ?????? = ?
Calculations of Specific Heat
Trial 1: qwater=(4.187J/g)(45.8g)(1.3C)= 249J
Cmetal= 20.7 oC -90.1 oC =-69.4 oC
-249J/(20.7g*-69.4 oC)= .173J/g oC
Trial 2: qwater=(4.187J/g)(47.7)(2.3C)= 459J
Cmetal= 20.2 oC -91.6 oC =-71.4 oC
-459J/(19.3g*-71.4 oC)= .319 J/g oC
Trial 3: qwater=(4.187J/g)(46.5g)(2.5 oC)= 487
Cmetal= 19.69 oC -89.3 oC =-69.61 oC
-487J/(20.2g*-69.6 oC)= .346 J/g oC
Average Specific Heat:
(.173+.319+.346)/3=.279
5. Putting it all together!
a. Using the table of Specific Heat Capacities in the Pre-Lab Handout as well as the average specific heat capacity you calculated in Question 4, what is the identity of the unknown metal? Explain, in 2 – 3 sentences, how you know.
b. If you repeated this experiment with a strip of aluminum with the same mass as the strip of metal you used, would the water heat up to a higher or lower
temperature using aluminum than it did for your experimental metal? Explain, in 2 – 3 sentences, your reasoning.
Hint: Compare the specific heat values of aluminum and your experimental metal.
6. Complete the following table with your experimental data for the cold pack and hand warmer experiments below. Highlight (in yellow) the minimum temperature for the cold pack and the maximum temperature for the hand warmer.
Tables 3 & 4
Cold Pack Hand Warmer
Time (sec) Temperature (°C) Temperature (°C)
Initial 21.7 26.0
30 15 32.5
60 10.4 39.0
90 9.5 43.5
120 8.3 47.9
150 7.2 57.0
180 5.7 60.6
210 4.6 61.0
240 4.1 64.5
270 4.1 68.7
300 3.9 70.6
330 3.8 70.9
Tables 3 & 4
Cold Pack Hand Warmer
Time (sec) Temperature (°C) Temperature (°C)
360 3.7 71.7
390 3.4 73.0
420 3.4 73.8
450 3.9 74.5
7. Plot your data from Tables 3 and 4 (TWO data sets from Question 6) on one x-y scatter plot using a graphing program. Your graph should show the temperature as a function of time. You will not receive credit if you draw your graph by hand. Excel or Google Sheets are good choices that you can learn how to use quickly if you don’t already have a favorite graphing program.
On your graph, the x-axis should be the time (in seconds), and the y-axis should be the temperature (in °C) at that time. Make sure to label the axes including the units in parentheses but REMOVE the chart title and any default legend put in by the graphing software.
In the figure legend (text below the graph), explain the data in your graph.
Be sure to use different symbols (and make them different colors) for the two data sets and mark the maximum (hand warmer) and minimum (cold pack) temperatures with an asterisk. Do NOT include a trendline or connect the data points.
See other general tips for making graphs in the How to Make a Graph in Excel document located in the Introductory Materials for this lab.
68.7 70.
61 64.5
57 60.6
43.5 47.9
39
32.5
15
10.4 9.5
8.3 7.2 5.7 4.6 4.1 4.1 3.9 3.8 3.7 3.4 3.4 3.9
8. Putting it all together! Consider the temperature changes experienced by the cold pack and the hand warmer and answer the questions below.
a. What was the overall ΔT (change in temperature) for the cold pack? Show your work and include units.
b. What was the overall ΔT (change in temperature) for the hot pack? Show your work and include units.
c. Which one worked via an endothermic process? Via an exothermic process?
Explain in 2 – 3 sentences.
d. Which pack had the greatest change in enthalpy? Explain, in 2 – 3 sentences, how you know using your experimental data.
[Show More]
Last updated: 3 years ago
Preview 1 out of 11 pages


.png)
















