Chemistry > QUESTIONS & ANSWERS > CH101 CHEMISTRY 101 Lab 9 PostLab - Titrations. Current Score 25 / 25 | North Carolina State Unive (All)
CH101 CHEMISTRY 101 Lab 9 PostLab - Titrations. Current Score 25 / 25 | North Carolina State University CH
Document Content and Description Below
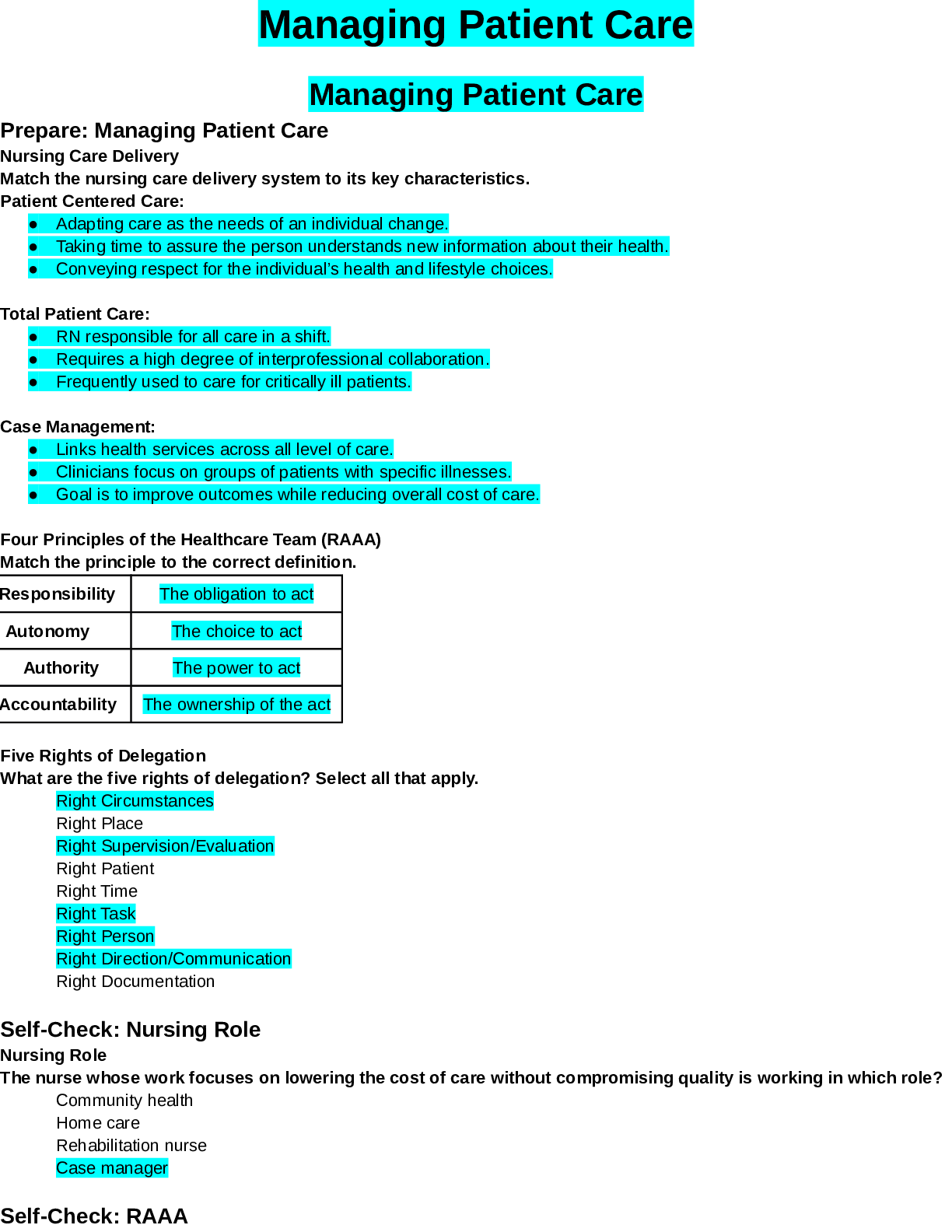
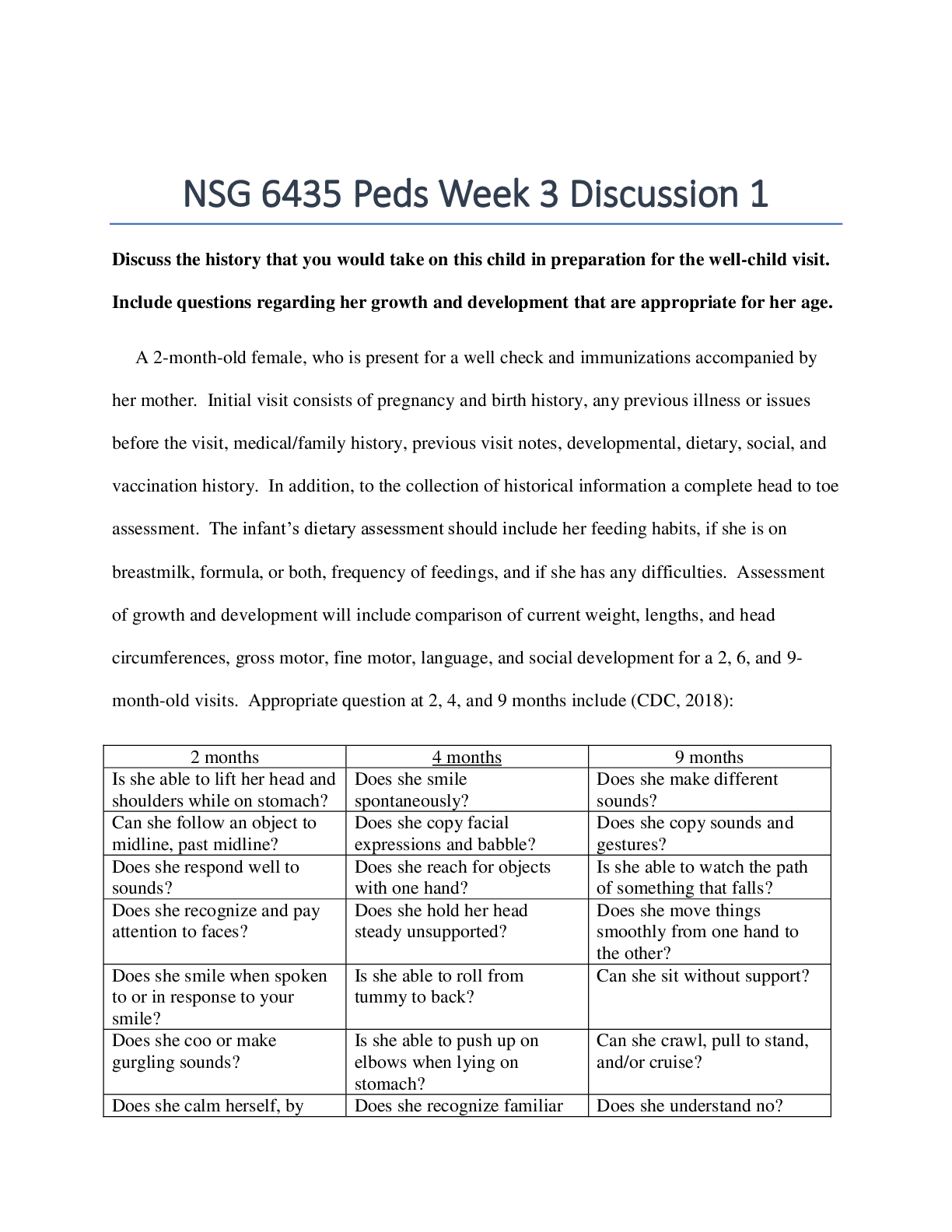
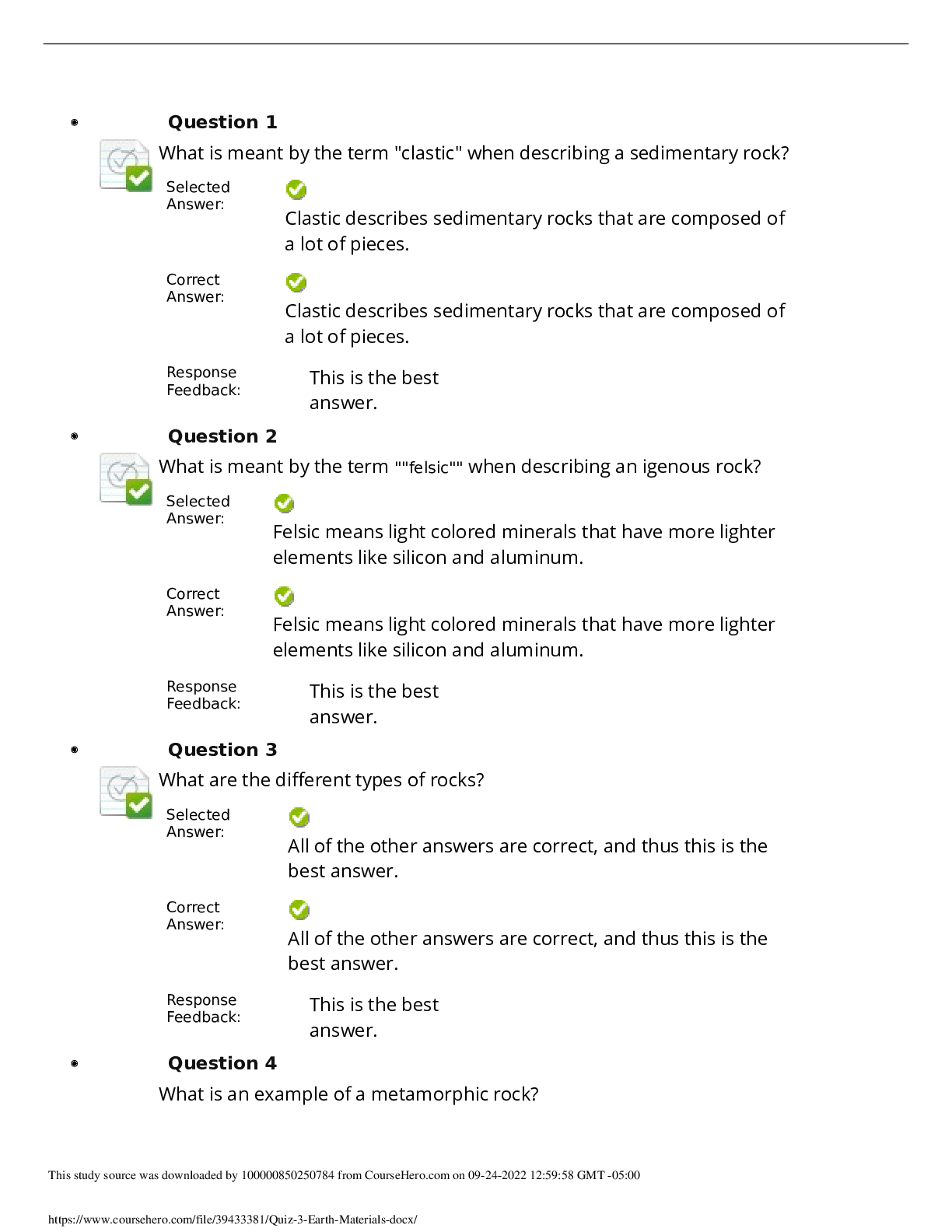
North Carolina State University CH CH101 CHEMISTRY 101 Lab 9 PostLab - Titrations Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/las... t?dep=10120923 Page 1 of 6 Current Score : 25 / 25 Due : Tuesday, November 11 2014 11:00 PM EST 1. 6/6 points | Previous AnswersNCSUGenChem102LabV1 9.POST.01. Consider a different titrant for this exercise. Suppose Ca(OH)2 were used as the titrant, instead of NaOH. This will make the titrant twice as concentrated in hydroxide ion. The analyte will still be HC2H3O2. (a) What is the stoichiometry of HC2H3O2 to Ca(OH)2? (b) Complete the following table for this titration. Data Table P1: Titration of acetic acid with calcium hydroxide concentration of Ca(OH)2 0.364 M volume vinegar solution 12.04 mL mass vinegar solution 12.10 g volume of Ca(OH)2 solution 12.70 mL mmol of Ca(OH)2 4.62 mmol Lab 9 PostLab - Titrations (Postlab) Benton Gorre CH 102, section 111, Fall 2014 Instructor: Pallavi Singh TA WebAssign The due date for this assignment is past. Your work can be viewed below, but no changes can be made. Important! Before you view the answer key, decide whether or not you plan to request an extension. Your Instructor may not grant you an extension if you have viewed the answer key. Automatic extensions are not granted if you have viewed the answer key. Request Extension View Key 8:3 3:8 18:25:43 GMT -05:00 Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120923 Page 2 of 6 mmol of HC2H3O2 9.24 mmol mass of HC2H3O2 .554 g mass % of HC2H3O2 in the original sample 4.58 % molarity of HC2H3O2 in the original sample .767 M Additional Materials Titrations Periodic Table 2. 6/6 points | Previous AnswersNCSUGenChem102LabV1 9.POST.02. Consider a different analyte for this exercise. Citric acid is found in many fruits and fruit juices. Sodium hydroxide (NaOH) is the titrant and citric acid (H3C6H5O7) the analyte according to the following balanced chemical equation. H3C6H5O7 + 3 OH- → C6H5O73- + 3 H2O (a) What is the stoichiometry of H3C6H5O7 to OH-? (b) Complete the following table for this titration. Data Table P2: Titration of citric acid in orange juice with sodium hydroxide. 3:8 18:25:43 GMT -05:00 Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120923 Page 3 of 6 concentration of OH- 0.0794 M volume orange juice 9.21 mL mass orange juice 9.36 g volume of OH- solution 25.79 mL mmol of OH- 2.05 mmol mmol of H3C6H5O7 .683 mmol mass of H3C6H5O7 .131 g mass % of H3C6H5O7 in orange juice 1.40 % molarity of H3C6H5O7 in orange juice .0742 M Additional Materials Titrations Periodic Table 3. 6/6 points | Previous AnswersNCSUGenChem102LabV1 9.POST.03. Consider a different titration for this exercise. Potassium permanganate (KMnO4) is the titrant and hydrogen peroxide (H2O2) the analyte according to the following balanced chemical equation. 2 MnO4- + 5 H2O2 + 6 H+ → 2 Mn2+ + 5 O2 + 8 H2O (a) What is the stoichiometry of MnO4- to H2O2? 18:25:43 GMT -05:00 Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120923 Page 4 of 6 (b) Complete the following table for this titration. Data Table P3: Titration of hydrogen peroxide with potassium permanganate. concentration of MnO 4 - 0.539 M volume H2O2 solution 17.98 mL mass H2O2 solution 18.06 g volume of MnO 4 - solution 16.55 mL mmol of MnO 4 - 8.92 mmol mmol of H2O2 22.3 mmol mass of H2O2 .758 g mass % of H2O2 in the original sample 4.20 % molarity of H2O2 in the original sample 1.24 M Additional Materials Titrations Periodic Table 4. 7/7 points | Previous AnswersNCSUGenChem102LabV1 9.POST.04. 8:3 Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120923 Page 5 of 6 Green checks and red X's are not displayed for this question. Indicators are weak acids or bases that have different colors in their acid and base forms. Chemists make use of these noticeable color changes by using them in acid-base titration experiments. The equivalence point of a titration is where the acid and the base have reacted in stoichiometric proportions. The end point of a titration is where the indicator changes color. Selecting an indicator that changes color at a point as close as possible to the equivalence point is crucial for accurate quantitative analysis using titration. (a) Consider the titration curve below and the provided table of indicators. Select the appropriate indicator(s) for the titration. indicator color change pH good for this titration? acidic form basic form Bromophenol Blue 3.0 - 4.6 Yes yellow purple Methyl Orange 3.1 - 4.4 Yes red orangeyellow Alizarin Red S 4.6 - 6.0 No yellow red Thymol Blue 8.0 - 9.6 No yellow blue Phenolphthalein 8.2 - 9.8 No colorless pink Alizarin Yellow R 10.2 - 12.0 No yellow red (b) Thymol Blue might be selected as an indicator for the titration of a weak acid with a strong base. Can you estimate the pH at the equivalence point? 18:25:43 GMT -05:00 Lab 9 PostLab - Titrations 11/25/14, 12:42 PM http://www.webassign.net/web/Student/Assignment-Responses/last?dep=10120923 Page 6 of 6 Additional Materials Titrations Yes. The equivalence point should be somewhere between a pH of 7.0 and 8.0. Yes. The equivalence point should be somewhere between a pH of 3.1 and 4.4. Yes. The equivalence point should be somewhere between a pH of 8.0 and 9.6. Yes. The equivalence point should be somewhere between a pH of 9.6 and 10.6. No. We would need to know the concentration of the titrant before we could do that. No. We would need to know the volumes of both the analyte and the titrant before we could do that. 18:25:44 GMT -05:00 [Show More]
Last updated: 9 months ago
Preview 1 out of 6 pages
IIUUKK.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$5.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Oct 06, 2022
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Oct 06, 2022
Downloads
0
Views
77