General Science > QUESTIONS & ANSWERS > Pennsylvania State University - MATSE 201quiz 1. 100% (All)
Pennsylvania State University - MATSE 201quiz 1. 100%
Document Content and Description Below
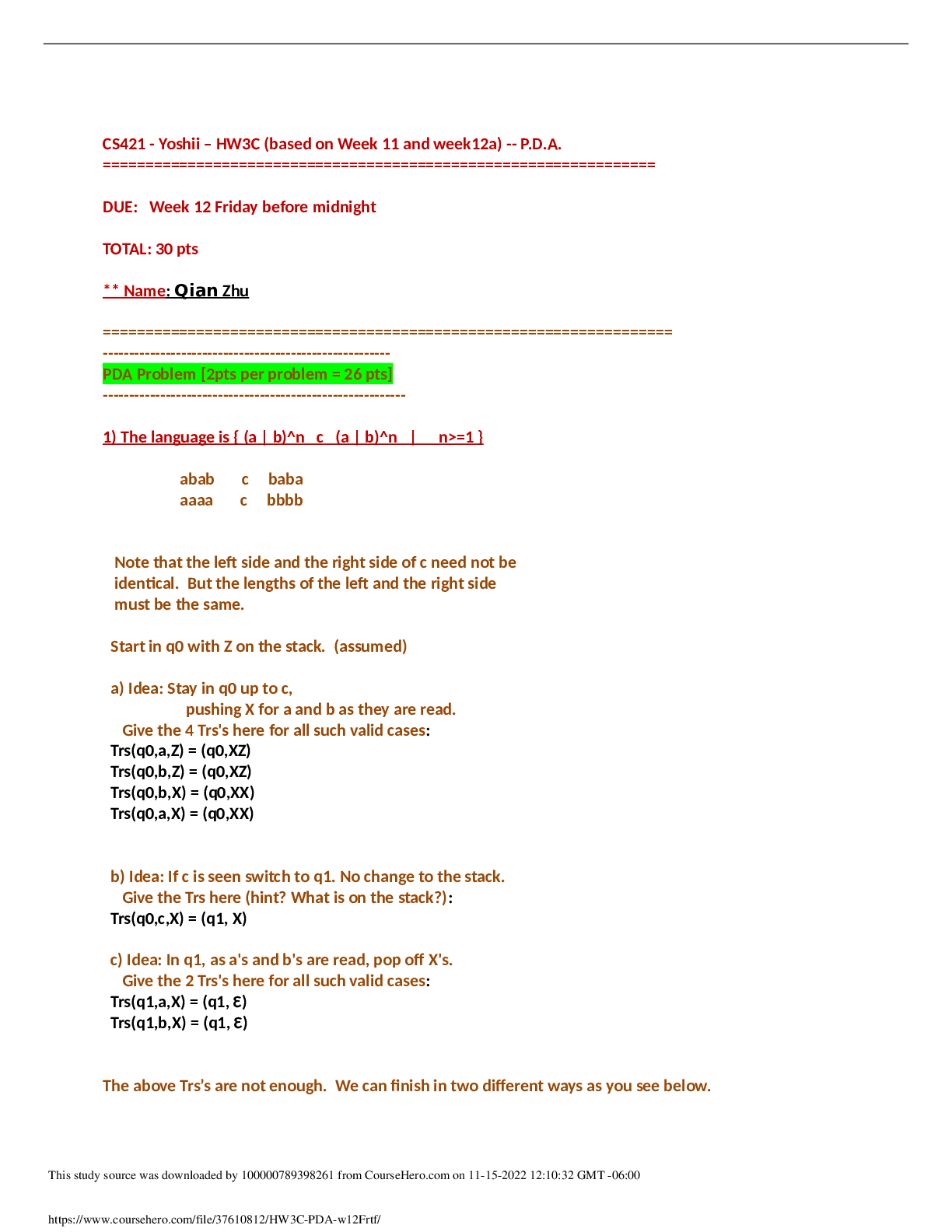
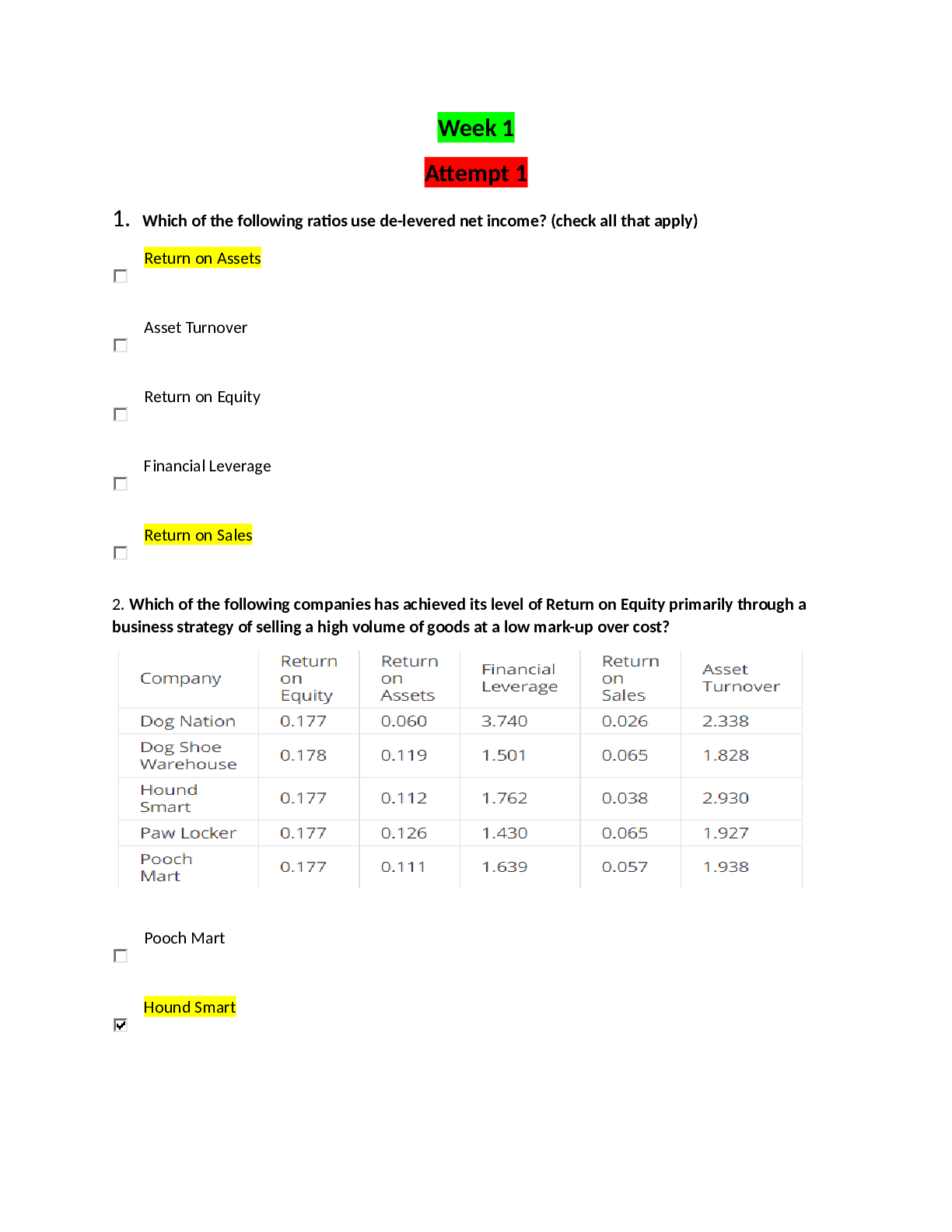
Pennsylvania State University - MATSE 201quiz 1 1. Ethane (CH3CH3) and Fluoromethane (CH3F) have the same number of electrons and are essentially the same size. However, ethane has a boiling... point of 184.5C and fluoromethane has a boiling point of 194.7C. Which answer best explains this 10 degree difference in boiling point in terms of the van der Waals forces present. A) It has nothing to do with van der Waals B) Both molecules have both dispersion and orientation forces present, but fluoromethane is a slightly larger molecule C) Both molecules have dispersion forces, but fluoromethane also has orientation forces present D) Both molecules are non-polar, but fluoromethane is a slightly larger molecule Table for Individual Question Feedback Points Earned: 2.0/2.0 2. Covalent interactions are generally long range (i.e., greater than 10 nm). A) True B) False Table for Individual Question Feedback Points Earned: 2.0/2.0 3. Hydrogen bonding is only found between polar molecules. A) True B) False Table for Individual Question Feedback Points Earned: 2.0/2.0 4. Describe all types of bonding that you would expect to exist in each of the following compounds: a silicon carbide crystal A) Predominantly covalent and some ionic B) Predominantly ionic and some covalent C) Equally ionic and covalent D) Ionic only Table for Individual Question Feedback Points Earned: 2.0/2.0 5. Describe all types of bonding that you would expect to exist in each of the following compounds: potassium fluoride (KF) A) predominantly covalent and some ionic B) ionic only C) covalent only D) predominantly ionic and some covalent Table for Individual Question Feedback Points Earned: 2.0/2.0 6. Describe all types of bonding that you would expect to exist in each of the following compounds: an alloy composed of 90%Al, 7%Si, 3%Mg A) ionic, covalent and metallic B) metallic only C) metallic and ionic D) metallic and van der Waals Table for Individual Question Feedback Points Earned: 2.0/2.0 7. Describe all types of bonding that you would expect to exist in each of the following compounds: zirconia (ZrO2) A) predominantly ionic and some covalent B) predominantly covalent and some ionic C) ionic only D) covalent only Table for Individual Question Feedback Points Earned: 2.0/2.0 8. Describe all types of bonding that you would expect to exist in each of the following compounds: ice A) predominantly ionic and some covalent B) van der Waals only C) predominantly covalent, some ionic and van der Waals D) predominantly ionic and some van der Waals Table for Individual Question Feedback Points Earned: 2.0/2.0 9. Covalent bonding occurs as a result of columbic attraction and the transfer of valence electrons. True or False? A) True B) False Table for Individual Question Feedback Points Earned: 2.0/2.0 10. Metallic bonds are different from covalent bonds in that the shared valence electrons are delocalized. True or False? A) True B) False Table for Individual Question Feedback Points Earned: 2.0/2.0 [Show More]
Last updated: 2 years ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 09, 2020
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Apr 09, 2020
Downloads
0
Views
122




.png)
.png)
.png)
.png)
.png)
.png)
.png)

