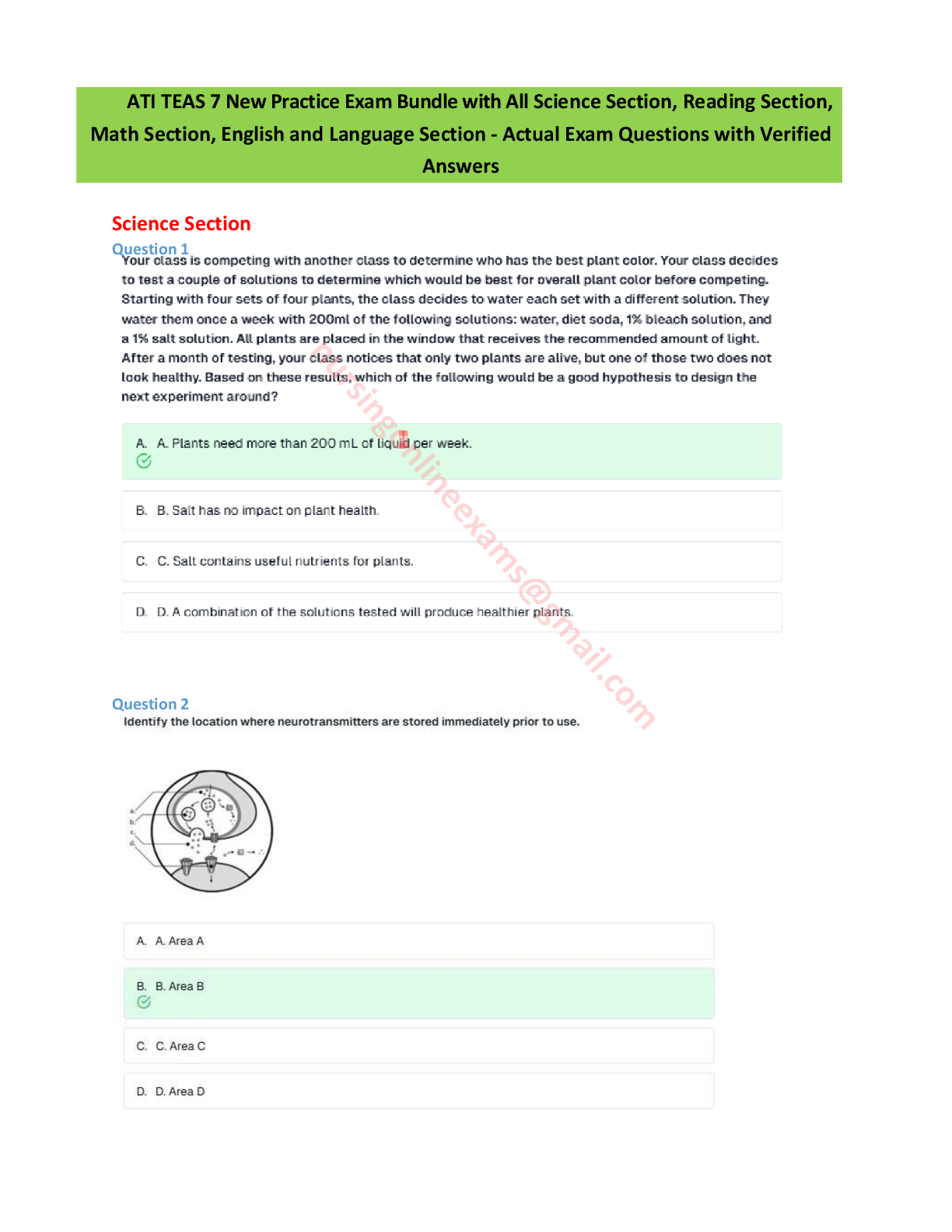
Foundations- Medications Exam review
Document Content and Description Below
. Medications - My
1. Discuss drug legislation in the US
- In 1906, the Pure Food and Drug Act designated the United States Pharmacopeia and the National Formulary as the official drug standards in
...
the United States,
- in 1938 the Federal Food, Drug and Cosmetic Act prohibited adulterated or mislabeled drugs from being made available.FDA enforces this law. Extensive testing of new drugs is required before marketing.
- in 1952 The Durham-Humphrey amendment to the Federal Food, Drug and Cosmetic Act distinguished prescription drugs from nonprescription (over-the-counter) drugs and provided directions for dispensing prescription drugs.
- The Kefauver-Harris Amendment of 1962 increased controls on drug safety, requiring tighter testing of drugs and written inclusion in the drug literature of adverse reactions and contraindications for approved drugs.
- The Comprehensive Drug Abuse Prevention and Control Act, also known as the Controlled Substances Act, was passed in 1970. This law regulates the distribution of narcotics and other drugs of abuse.
- In 1983, the Drug Enforcement Administration (DEA), a part of the Department of Justice, was identified as the nation’s sole legal drug enforcement agency.
- More current drug-related legislation= the Food and Drug Administration Modernization Act of 1997 provides for accelerated review and use of new drugs and approves drug testing in children before marketing.
- In 2003, the Pediatric Research Equity Act was signed into legislation. This act authorizes the FDA to require testing by drug manufacturers of drugs and biologic products for their safety and effectiveness in children
- Also in 2003, Congress approved the Medicare Prescription Drug Improvement and Modernization Act (MMA). This legislation provides financial assistance to seniors to purchase needed prescription medications.
2. Describe basic principles of pharmacology, including drug nomenclature and types of drug preparations.
- chemical name is a precise description of the drug’s chemical composition, identifying the drug’s atomic and molecular structure using exact chemical language and terminology.
- generic name, which identifies the drug’s active ingredient, is the name assigned by the manufacturer that first develops the drug
- official name is the name by which the drug is identified in the official publications, United States Pharmacopeia and National Formulary (USP and NF). The official name is often the generic name.
- trade name, also referred to as the brand name or proprietary name, is selected by the pharmaceutical company that sells the drug and is protected by trademark.
→ Nurses should be familiar with a drug’s generic and trade names.
● TABLE 28-1 Common Types of Drug Preparations
PREPARATION DESCRIPTION
Capsule Powder or gel form of an active drug enclosed in a gelatinous container; may also be called liquigel
Elixir Medication in a clear liquid containing water, alcohol, sweeteners, and flavor
Enteric coated A tablet or pill coated to prevent stomach irritation
Extended release Preparation of a medication that allows for slow and continuous release over a predetermined period;
Liniment Medication mixed with alcohol, oil, or soap, which is rubbed on the skin
Lotion Drug particles in a solution for topical use
Lozenge Small oval, round, or oblong preparation containing a drug in a flavored or sweetened base, which dissolves in the mouth and releases the medication; also called troche
Ointment Semisolid preparation containing a drug to be applied externally; also called an unction
Pill Mixture of a powdered drug with a cohesive material; may be round or oval
Powder Single or mixture of finely ground drugs
Solution A drug dissolved in another substance (e.g., in an aqueous solution)
Suppository An easily melted medication preparation in a firm base such as gelatin that is inserted into the body (rectum, vagina, urethra)
Suspension Finely divided, undissolved particles in a liquid medium; should be shaken before use
Syrup Medication combined in a water and sugar solution
Tablet Small, solid dose of medication, compressed or molded; may be any color, size, or shape; enteric-coated tablets are coated with a substance that is insoluble in gastric acids to reduce gastric irritation by the drug
Transdermal patch Unit dose of medication applied directly to skin for diffusion through skin and absorption into the bloodstream
3. Develop an understanding of basic principles of pharmacology, including mechanisms of drug action, adverse drug effects, and factors affecting drug action.
A. Pharmacokinetics = the effect of the body on the drug, once the drug enters the body.
● ABSORPTION = process by which a drug is transferred from its site of entry into the body to the bloodstream, influenced by the following factors.
❏ Route of Administration (orally usually take the longest, injected medications are usually absorbed more rapidly)
❏ Lipid Solubility (Cell membranes have a fatty acid layer. A drug that is more lipid soluble can be absorbed more readily and pass more easily through)
❏ pH (Acidic drugs are well absorbed in the stomach)
❏ Blood Flow (Absorption is increased with increased blood flow)
❏ Local Conditions at the Site of Administration (The more extensive the absorbing surface, the greater the absorption of the drug and the more rapid the effect. Contact time also affects absorption)
❏ Drug Dosage
i. A loading dose, or a larger than normal dose, is usually given when a patient is in acute distress and the maximum therapeutic effect is desired as quickly as possible.
ii. A maintenance dose is a lower dosage that becomes the usual or daily dosage.
● DISTRIBUTION = drug is distributed throughout the body, becoming available to body fluids and body tissues, depending on blood flow to the tissues, the drug’s ability to leave the bloodstream, and the drug’s ability to enter the cells
- drug may bind to plasma proteins, which causes unequal distribution and prevents the drug from reaching its intended site of action.
- blood–brain barrier prevents toxins and poisons from reaching the brain. Unfortunately, many drugs cannot penetrate the barrier, obviously influencing distribution.
- The placenta, on the other hand, is an ineffective barrier. Drugs readily move across the placenta, and many drugs produce harmful effects in the fetus.
● METABOLISM (biotransformation) = the change of a drug from its original form to a new form. The liver is the primary site for drug metabolism
- First-pass effect (Drugs move from the intestinal lumen to the liver by way of the portal vein and DO NOT go directly into the systemic circulation following oral absorption)
● EXCRETION = the process of removing a drug, or its metabolites (products of metabolism), from the body. The kidneys excrete most drugs. The lungs are the primary route for the excretion of gaseous substances, such as inhalation anesthetics. Many drugs are excreted through bile in the gastrointestinal tract. The sweat, salivary, and mammary glands are also routes of drug excretion.
- Some medications may be contraindicated, or dosages may need to be adjusted, if renal excretion is impaired.
- Manufacturers are required by law to include specific information regarding implications for geriatric patients on the package inserts of certain drugs. This law affects psychotropic drugs, nonsteroidal anti-inflammatory agents (NSAIDs), oral hypoglycemic agents, anticoagulants, certain broad-spectrum antibiotics, and cardiac drugs.
B. Pharmacodynamics = process by which drugs alter cell physiology and affect the body is called pharmacodynamics
- Drugs act at the cellular level to achieve the desired effect
- Mechanism of drug action is a drug–receptor interaction, The drug fits the receptor as a key fits a lock.
- A drug may combine with an enzyme to achieve the desired effect, which is referred to as a drug–enzyme interaction.
- Other drugs act on the cell membrane or alter the cellular environment to achieve their effect.
C. ADVERSE DRUG REACTIONS:
a. Some are predictable and may be tolerated as part of the therapy. (Eg: A known adverse effect with morphine use is constipation)
b. It can be severe and may require discontinuation of the drug, depending on whether the benefit of the drug outweighs the harm from the adverse effect.
c. Development of an iatrogenic disorder caused unintentionally by drug therapy. (Eg: Neutropenia caused by chemotherapy
d. Allergic Effect = an immune system response that occurs when the body interprets the administered drug as a foreign substance and forms antibodies against the drug. S&S rash, urticaria, fever, diarrhea, nausea, and vomiting. The most serious allergic effect is called an anaphylactic reaction (anaphylaxis).
e. Drug Tolerance = occurs when the body becomes accustomed to the effects of a particular drug over a period of time. Larger doses of the drug must be taken to produce the desired effect.
f. Toxic Effect = occur from a cumulative effect. A cumulative effect occurs when the body cannot metabolize one dose of a drug before another dose is administered. The drug is taken in more frequently than it is excreted, and each new dose increases the total quantity in the body.
- (Eg: Older patients are at risk for experiencing a cumulative effect, related to altered drug metabolism and elimination due to impaired hepatic metabolism and renal clearance related to normal changes with aging.)
[Show More]
Last updated: 3 years ago
Preview 1 out of 48 pages