Updated (2022) Comprehensive Exam for the Certification of Pharmacy Technicians/ Exam for the Certification of Pharmacy Technicians (ExCPT). Over 700 QnA.
Document Content and Description Below
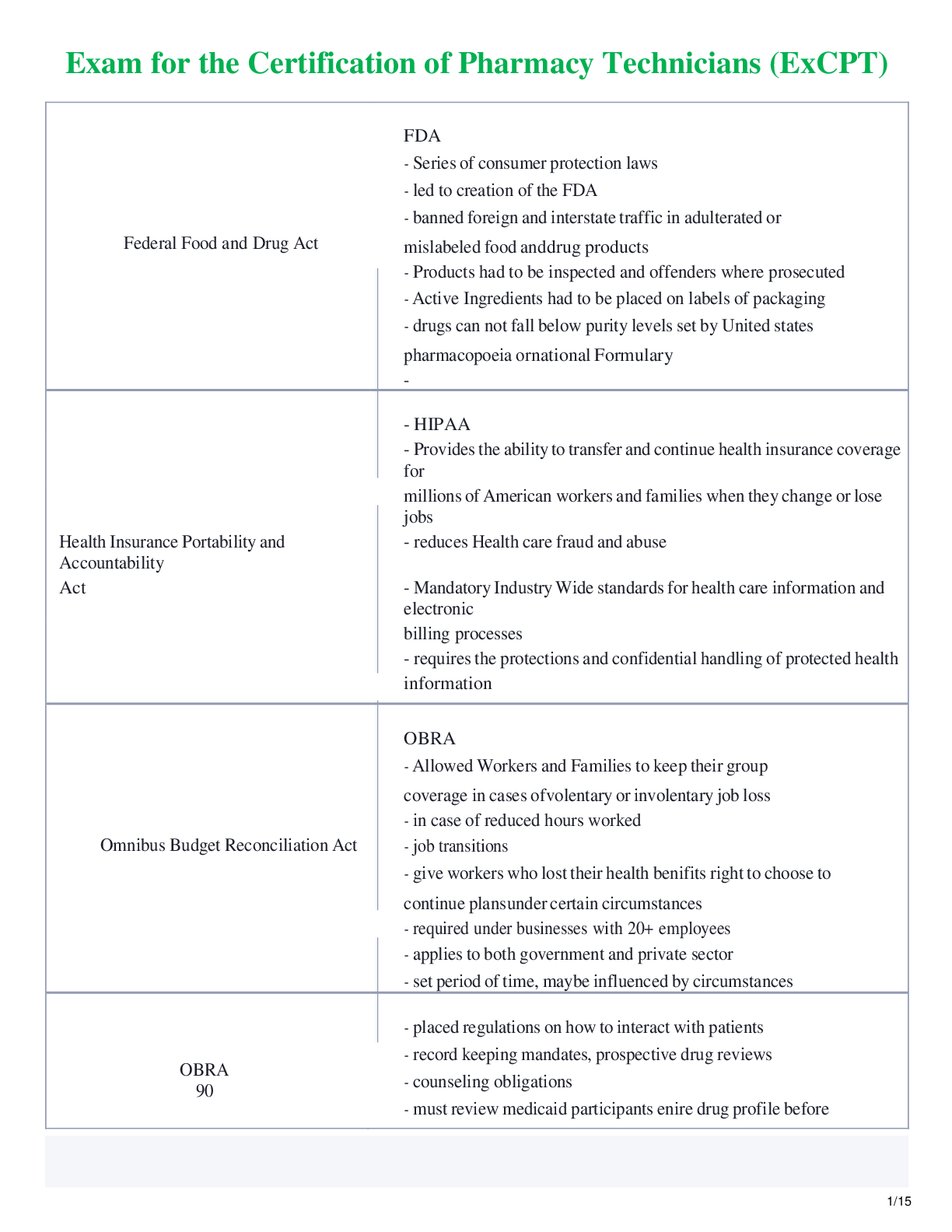
Federal Food and Drug Act
FDA
- Series of consumer protection laws
- led to creation of the FDA
- banned foreign and interstate traffic in adulterated or mislabeled food and drug products
- Produ
...
cts had to be inspected and offenders where prosecuted
- Active Ingredients had to be placed on labels of packaging
- drugs can not fall below purity levels set by United states pharmacopoeia or national Formulary
-
Health Insurance Portability and Accountability Act
- HIPAA
- Provides the ability to transfer and continue health insurance coverage for millions of American workers and families when they change or lose jobs
- reduces Health care fraud and abuse
- Mandatory Industry Wide standards for health care information and electronic billing processes
- requires the protections and confidential handling of protected health information
Omnibus Budget Reconciliation Act
OBRA
- Allowed Workers and Families to keep their group coverage in cases of volentary or involentary job loss
- in case of reduced hours worked
- job transitions
- give workers who lost their health benifits right to choose to continue plans under certain circumstances
- required under businesses with 20+ employees
- applies to both government and private sector
- set period of time, maybe influenced by circumstances
OBRA 90
- placed regulations on how to interact with patients
- record keeping mandates, prospective drug reviews
- counseling obligations
- must review medicaid participants enire drug profile before prescribing medications
Medicaid
- Social Health care program for families and individuals with low income + recources
- government insurance for people of all ages who can not afford sufficient health care
- varies from state to state --> joint funded between state and federal
- must be US citizens or legal permanent residents
-
Medicare
- National Social Insurance Program
- administered by US government
- provides health insuracne for Americans aged 65+
- members must have worked and payed for the system
- provides health insurance for younger people with disabilities
-
Disability
- Form of Insurance that insures the beneficiaries income against the risk that disability creates a barrier for a worker to complete the core functions of their work
- encompasses paid sick leave, short term disability benefits, and long term disability benifits
-
U.S. Social Security Act
- Social Welfare Legislation Act
- provides federal assistance to those who can not wotk
-
Combat Methamphetamine Epidemic Act
- enacted to curb the growing problem of underground manufacture of illegal drugs
- regulates over the counter products that can be used to make drugs
- include daily sales limits and 30 day purchase limits
- places products out of easy reach
- sale logbooks
- customer ID verification
- training of employees and sellers
-
Sale of Ephedrin
- sale is limited to behind the counter
- must present photo ID
- stores require to keep personal information on buyers for at least 2 years
- sales allowed in drive through lanes
U.S. Merck + co
- An American pharmaceutical company
- one of the largest in the world
- publishes Merck Manuals --> a series of reference books for medical professionals
- Merck Index -->list of chemical compounds --> lost
Poison prevention Packaging Act
PPPA
- requires use of child resistant packaging for prescription drugs, OTC, household chemicals, and hazerdous materials
- some drugs are exempt from child proof packaging --> OTC drugs with the proper labeling not for children and in big enough packages
Kefauver - Harris Act
- requires drug manufacturers to provide proof of their effectivness and safety before approval
- requires drug advertisements to disclose side effects
- stopped cheap generic drugs from being marketed as more expensive drugs and taking credit as breakthrough medications
Controlled Substances Act
CSA
- federal US drug Policy
- manufacture, importation, possession, use and distribution of certain substances is regulated
- created 5 classifications of schedules
- all controlled substances need to be inventoried Biannually
Schedule I Drugs
- High Potential for abuse
- no potential medical use or treatment in US
- lack of accepted safety for use of drug under medical supervision
- No prescriptions may be written for drug
- Crimes involving drugs can be quite serious
Schedule II Drugs
- Drugs have high potential for abuse
- Drugs have currently accepted medical uses, although there may be serious restrictions
- Abuse of drug may lead to severe psychological or physical dependence
- may not be used unless directly dispensed by a practitioner
- refills not allowed --> must write multiple prescriptions at once
- must be stored in a safe, while other schedules can be stored throughout pharmacy
Schedule III Drugs
- has potential for abuse, but to a lesser degree than schedules I + II
- drug has an accepted medical treatment
- abuse of drug may lead to moderate or low physical dependency or high psychological dependence
- may not be dispensed without a prescription
- may not be dispensed six months after prescription written
- may not be refilled more than 5 times
- may be prescribed orally or over phone
Schedule IV Drugs
- low potential for abuse
- has currently accepted medical use
- abuse may lead to limited physical or psychological dependence
- can be refilled up to five times after 6 months written or orally
Schedule V Drugs
- low potential for abuse
- currently accepted medical use
- limited dependence
- may be dispensed without medical reason
- orally or written
DEA form 222 1 + 2 order form
- Order Forms for Schedule 1 and 2 drugs
- used to order from another registrant retail and hospital pharmacies
- wholesaler or retailers use this form
- forms need to be maintained for 2 years --> all forms
Standard Invoice
...
DEA form 69
...
DEA Form 41 form of controlled substances
inventory and destruction
- registers inventory of controlled substances
- used to return drugs to DEA or acceptable recipients
- documents surrender or destruction of controlled substances
- forwarded to DEA for disposal
DEA 82 form of inspection
- Notice of Inspections of controlled premises
- informs registrant an inspection will be made
DEA form 104 surrender and close
- Voluntary surrender of controlled substance privileges
- used to surrender permit to the DEA on a voluntary basis
- used to close pharmacys
DEA 106/666
loss or theft
- report loss or theft of controlled substances
- Report discovered shortage of controlled substances
DEA 222a
- used to obtain DEA 222 form from DEA
DEA 224 license form - institutions
- new Application for registration of controlled substances act
- Form used by retail pharmacies, clinics, hospitals, practitioners, and teaching institutions,
- to get license to obtain or dispense controlled substances
DEA 225 license form sellers
New application for registration used by manufacturers, distributors , wholesalers, importers, exporters, researchers
- to obtain and use controlled substances
DEA 226
application for re-registration of controlled substances
- privileged by retail pharmacies, hospitals, practitioners or teachers to obtain or dispense controlled meds
DEA 363 narcotics for pharmacy programs
- Registration for narcotic rx programs
- used to obtain approval narcotic rx program using controlled substances
Ordering and recipt of samples
Only a practicioner who has been issued individual DEA numbers are authroized to recieve any controlled substances in a sample, starter pack, or any container
- Only those with industrial prescribing authority are allowed to order / receive any dangerous drug sample/starter pack
Insulin Storage
- recommended temperature is 36 - 46 Farenheit --> unopened --> till expiration date
- Vials and cartiridges should be 59 - 86 up to 28 days
- if altered only two weeks
- loses effectivness when exposed to extreme temperatures or sunlight
- keep as cool as possible, but not frozen
-
Capsule sizes
- size 5 is the smallest --> bigger numbers are smaller
- su07 is the largest
- length and diameter increase as number goes down
- starts over again at 13 and goes down to 7
- four types of 0 in the middle
- two 7s (su07) and 2 12s (12el)
Capsule Information
- both types of capsules made from aqueous gelling agents --> mainly gelatin but sometimes cellulose
-
Hard Shelled Capsules
- made of gelatin
- contain dry powdered ingredients
- made in two halves --> lowered body is filled
- then sealed by higher diameter cap
Soft Shelled Capsules
- primarily used for oils and active ingredients that are dissolved or suspended in oil
- Single piece encapsulating method
- sealed with a single drop of gelatin
-
DEA numbers
- assigned to health care providers by the DEA allowing them to write prescriptions for controlled substances
- legally used solely to track controlled substances
- Also used by the Industry as general prescriber numbers -->unique identifier for anyone who can prescribe medications
-
DEA sections
- Contains 2 letters and 6 numbers and 1 check digit
- First Letter is code identifying type of registrant --> letters A - X depending on job type
- Second Letter is the first letter of the registrants last name
- seventh number is the checksum
Seventh number, check sum
- sum of digits 1, 3, 5
- then sum the digits of 2, 4, 6 and multiply by 2
- add the two sums together = CHECK
- Right most digit of the CHECK number is the 7th digit
MSDS
material safety data sheet
- material safety data sheet
- component of product stewardship, occupational safety and health, and spill handling procedures
- catalog information on chemicals, compounds, and mixtures
- info on safe use and potential hazards
- should be available for reference where chemicals are being stores
- for work settings
Drug Drug interactions
- when ever two or more drugs are taken, there is a chance that there will be interactions
- may increase or decrease the effects of intended uses and side effects
- more drugs taken more likely interactions will occur
-
Ways drug drug interactions occur
- absorption rate into the body
- distribution of drug throughout the body
- alteration made to drug in body --> metabolism
- elimination of drug from the body
Contraindicated
- Never take these drugs together
- high risk for dangerous interaction
- contra bad
Serious
- Potential for serious interaction
- regular monitoring by doctor is required
- alternate medicine may be necessary
- monitering by serious black
Significant
- Potential for significant interaction
- monitoring by doctor likely required
Minor
- Interaction is unlikely, minor, or insignificant
A
- Depreciated
- used by some older entities
B
Hospital/clinic
C
Practitioner
D
Teaching Institution
E
Manufacturer
F
Distributor
G
Researcher
H
Analytical Lab
J
Importer
K
Exporter
L
reverse Distributor
M
Mid level Practitioner
P, R, S, T, U
Narcotic Treatment Program
X
Suboxone/Subutex Prescribing Program
First Shift
7am - 3pm
- morning shift
Second Shift
3pm - 11pm
- afternoon shift
- also called swing shift
third shift
11pm - 1am
- night shift
Medication carts
- distributed from pharmacy throughout the facility
- 24 hours worth of medication included in each cart
-
FDA Class I Recall
- Dangerous products that could cause serious health problems or death
- contain toxins, allergens, label mix ups, defects
FDA Class II Recall
- for products that might cause a temporary health problem
- pose slight threat of serious nature
-
FDA Class III Recall
- For products unlikely to cause any adverse health reactions
- still violate FDA labeling and manufacturing laws
- container defects, lack of proper labeling, wrong color/taste
FDA Class Recalls
- company can call a recall on their own after discovering flaw
- FDA can call a recall
- if recall is not done FDA can call a seizure of product
- must quickly remove products that are potentially dangerous from the market
- Firms must take full responsibility for removal of dangerous products
-
Drug Expiration Guidelines
- Final day the manufacturer guarantees the drug to perform at full potency and safety
- exist on medication labels --> required by law
- no recommendation about drug given after expiration
- drugs typically last 12 - 60 months
- after container is opened, expiration no longer reliable
- shelf life may actually be longer than expiration date
- If just the month and year are indicated --> drug can be used or dispensed until the last day of that month
Strenght of solutions
- need to be calculated in grams/ml
Business days
- do not include weekends
- day an order arrives does not count as a business day
- do not include federal holidays
-
AMA
- American Medical Association
- largest associations of physicians and medical students in US
- promote art and science of medicine to better public health
- advance interests of physicians and patients and lobby for favorable legistlation
- raise money for medical education
- Publishes JAMA, Largest circulation of any weekly medical journal
PFC
- Pharmacy Formulary commitee
- lists all mediciens
- annualy updates drugs and ingredients
- standards must be met for strength, quality, and purity
HPhA
- Hospital Pharmacist Association
- Professional organization that represents the interests of pharmacists who practice in hospitals, health maintanence, long term care facilities, home care
-
P&T
- Pharmacy and Therapeutics commitee
- comittee at a hospital or insurance plan that meets to decide what drugs will appear at that entities drug formulary
- consists of physicians and pharmacists
- weigh cost benifits of each drug and decide which to use
NDC
- National Drug Code
- unique product identifier for drugs intended for human use
- registered drug establishments must provide FDA with a current list of all drugs manufactured
- 10 digit, 3 segment numeric identifier assigned to each medication
- once an NDC is assigned to a product, it may not be reused
- sometimes referred to as the drug listing act of 1972 - for coding
NDC segment 1
- first segment is the labelor code
- 4 or 5 digits
- assigned by FDA upon submission of a labelers code request
- labeler is any form that manufactures, repacks, or distributes a drug product
Second segment
- Product Code
- 3 or 4 digits long
- identifies a specific strength, dosage form, and formulation for a particular firm
- assigned by the labeler
third segment
- Package code
- 1 or 2 digits long
- identifies package forms and sizes
- assigned by the labeler
Orange Book
- Approved drug products with therapeutics equivalence evaluations
- identifies drugs approved on basis of safety and effectiveness by FDA
- lists patents
Red Book
- Multi volume treatise outlined fiscal law
- pricing and product information for drugs
- answers commonly asked question
Therapeutic Equivalence Index
- for generic drugs
PDR
Physicians Desk Reference
- commercially published compilation of manufacturers prescribing information, package inserts on prescription drugs
- provides physicians with list of fully mandated information for writing prescriptions
- widely available in libraries and bookstores
-
Auxiliary labels
For the X
- easily identifiable stickers --> bright colors with easily identifiable graphics
- available in Spanish and french
- contains warnings, Instructions for route of admission, Dietary information
- short to the point phrases
PTCB Certification
- every 2 years a minimum of 20 hours of continued education is neceassary
- 1 hour must be on patient safety
Min/Max inventory size
- the min is the numerator --> once point is reached a new order is needed to be placed
- max is denominator --> what the new order would reach up to
PPPA exception
- nitroglycerin SL
- aspirin or acetaminophen -- powder or tablets
isosorbid dinitrate
- erythromycin ethyl succinate
- potassium supplements
- sodium flouride
- betamethasone
- oral contraceptives
- pancrelipase
- anhydrous cholestyramine
- hormone replacement therapy
- sucrase
- estrogen tablets
- https://quizlet.com/10156794/pppa-exemptions-flash-cards/
Medicare Part A
- Hospital Insurance covers most medically necessary hospital care, skilled nursing facilities, home health and hospice care
- Free if you have worked and paid social security for more than 10 years (40 calender quarters)
- if less than montly premium will be paid
Medicare Part B
- Medical Insurance covers most medically necessary doctor, preventative care, medical equipment, hospital outpatient services, lab tests, mental health care, and home + ambulance
- always pay montly premium
Medicare Part C
- Part of policy that allows private insurance companies to provide Medicare benifits
- not a seperate benifit
- include HMOs and PPOs
- known as Medicare Advantage plans
Medicare Part D
- outpatient prescription drug insurance
- provided only through private insurance companies that have contacts with the government
- never directly provided by the government
- must choose coverage that works with Medicare benifits
Durham - Humphrey Amendment
- Defined two specific kinds of medications
- Legend drugs ( Prescriptions
- Over the counter Drugs
- legend drugs include drugs with habit forming or harmful side effects
tall Man lettering
Practice of writing part of a drugs name in upper case letters to help distinguish drugs that sound and look alike
- to avoid errors
- only for portion of drug name that are similar
[Show More]
Last updated: 3 years ago
Preview 1 out of 19 pages



























 (1).png)