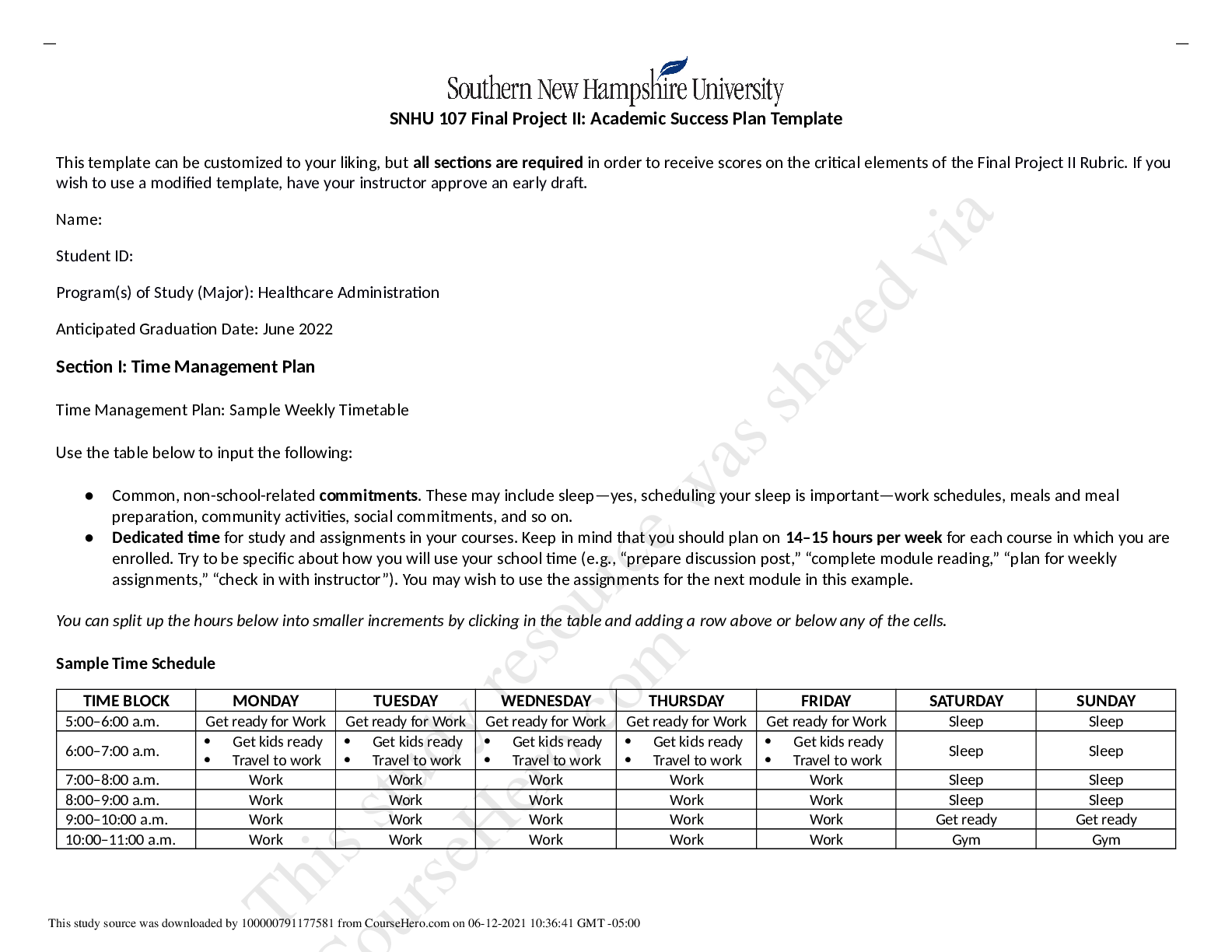
NR508_MIDTERM_EXAM_REVIEW ( A guaranteed)
Chapter 5: Adverse Drug Reactions
Mechanistic Classification of ADRs including Types of Immune-Mediated ADRs and Types A-F
- There are two basic types of ADRs: pharmacological
...
NR508_MIDTERM_EXAM_REVIEW ( A guaranteed)
Chapter 5: Adverse Drug Reactions
Mechanistic Classification of ADRs including Types of Immune-Mediated ADRs and Types A-F
- There are two basic types of ADRs: pharmacological and idiosyncratic
A pharmacological reaction is often predictable based on the drug's mechanism of action and is typically dose related. These reactions are often an exaggerated physiological response related to the pharmacology of the drug, for example, hypotension from the beta blocker metoprolol, diarrhea from the fat-blocking drug orlistat, and insomnia from the stimulant methylphenidate. These adverse reactions are often managed by withdrawing the medication or reducing the dose. Pharmacological ADRs may also occur based on secondary pharmacology, such as weight gain from the atypical antipsychotic olanzapine, flatulence from the fiber supplement psyllium, or myopathy from the HMG Co-A reductase drug simvastatin.
- Idiosyncratic reactions are unpredictable and may be more likely to result in mortality. Idiosyncratic reactions are mediated by the immune system, receptor abnormalities, drug–drug interactions, abnormalities in drug metabolism, pharmaceutical variations, or unmasking of an abnormal biological system. Most commonly, idiosyncratic reactions are mediated by the immune system when a drug molecule is recognized as a foreign substance.
Immune-Mediated Adverse Drug Reactions
Type I IgE-mediated, immediate-type hypersensitivity
Example: angioedema and anaphylaxis
Type II Antibody-dependent cytotoxicity
Example: heparin-induced thrombocytopenia
Type III Immune complex hypersensitivity
Example: Arthus reaction to tetanus vaccine
Type IV Cell-mediated or delayed hypersensivity
Example: Drug Rash, Eosinophilia and Systemic Syndrome
ADRs have also been categorized as Types A–F.
-Type A reactions are equivalent to pharmacological reactions, are dose-dependent, and are predictable
- Type B reactions are idiosyncratic, are not dose-dependent, and are not predictable
-Type C reactions result from chronic medication use
- Type D reactions are delayed
- Type E reactions are from drug–drug interactions
- Type F reactions result from treatment failures
Time-Related Classification of ADRs including drugs associated with withdrawal symptoms
- Rapid reactions occur during or immediately following the administration of a medication. These unintended adverse reactions generally occur when medications are administered improperly and are not necessarily related to being the first dose. For example, vancomycin can cause an adverse reaction known as red man syndrome when administered too rapidly (Aronson & Ferner, 2003). Phenytoin can cause can adverse reaction known as purple glove syndrome (blood vessel irritation and inflammation) when administered peripherally (Earnest, Marx, & Drury, 1983). Skin or tissue necrosis secondary to extravasation may occur with administration of chemotherapeutic agents. For example, hand-foot syndrome may occur when extravasation of chemotherapy occurs and damages the surrounding tissues in the hands and feet, causing redness, swelling, burning, blisters, ulcers, peeling skin, and difficulty when walking
- First-dose reactions occur following the first dose of a medication. For example, orthostatic hypotension is a common reaction that occurs following the first dose of doxazosin, which generally does not occur with repeated doses. Cytokine release syndrome can occur following the first dose of orthoclone OKT3. Patient education and monitoring are essential when administering medications that are known to have a first-dose reaction, especially to ensure continued adherence, as the reaction is unlikely to persist.
- Early reactions occur early in treatment and generally resolve with continued treatment as the patient develops tolerance. These reactions typically do not require discontinuation of the drug but may simply require patients to adapt to the medications. It is often useful to initiate drugs likely to cause early reactions with low starting doses and sequentially titrate the dose upward to mitigate the severity and duration of side effects. Examples include gastrointestinal upset following the initiation of metformin or selective serotonin reuptake inhibitors. Immune hypersensitivities may occur following the first or subsequent dose. These reactions, however, often do require immediate discontinuation of the drug and possibly further medical attention, such as in the case of anaphylaxis resulting from administration of penicillin or its derivatives.
- Intermediate reactions occur following repeated exposure to a medication. Examples include hyperuricemia from furosemide, hemolytic anemia from ceftriaxone, interstitial nephritis from penicillin G, and contact dermatitis from neomycin. Intermediate reactions are difficult to predict but should be monitored. Patients with predisposing factors or increased susceptibility for adverse reactions should be followed vigilantly while on therapy for occurrence of these reactions
- Late reactions occur after prolonged exposure to an offending agent. Examples include osteoporosis or thinning of the skin due to prolonged corticosteroid use or hypogonadism following prolonged use of opioids. It may be possible to symptomatically treat late adverse drug reactions, but most are predictable and occur following repeated exposures. Thus, it is often recommended to remove the offending agent before the reaction is predicted to occur in order to manage this type of adverse effect. Late reactions also include reactions that occur when a dose of a chronic medication is reduced or withdrawn. For example, rapid discontinuation of oxycodone can cause the patient to experience symptoms of withdrawal (i.e., anxiety, insomnia, rhinorrhea, diaphoresis, tremor, vomiting, diarrhea, and/or tachycardia). A patient who takes clonidine or propranolol may experience rebound hypertension following withdrawal of the medication. These types of adverse drug reactions are relatively common and can often be avoided by thoughtful tapering of the drug, as they are a predictable extension of the drug's therapeutic effect.
- Delayed reactions occur at variable time points following drug exposure and can even occur after the discontinuation of a drug. For example, drug-induced tardive dyskinesia may occur following prolonged exposure to antipsychotics or metoclopramide, with symptoms persisting for months to years following discontinuation of the precipitating drug. Polyalkylimide implant injection (cosmetic filler) can cause swelling and tender nodules near the injection site as well as other symptoms (fever, arthritis, xerostomia) up to 12 months following the injection
Dose-Related ADRs classification
-Adverse drug reactions may also be dose-related could be from administering an excessive dose or failing to adjust doses properly for age and organ function (i.e., renal insufficiency or liver failure). For example, a person with diabetes may become hypoglycemic after administering an excessive dose of insulin. An individual patient could experience lithium toxicity upon development of acute renal failure with no adjustment of the lithium dose.
Severity of ADRs
[Show More]