Engineering > Lab Report > MSE 235 XRD of Metals and Crystal structure Lab template F15 2020 – Purdue University | MSE235 XRD (All)
MSE 235 XRD of Metals and Crystal structure Lab template F15 2020 – Purdue University | MSE235 XRD of Metals and Crystal structure Lab template F15 2020
Document Content and Description Below
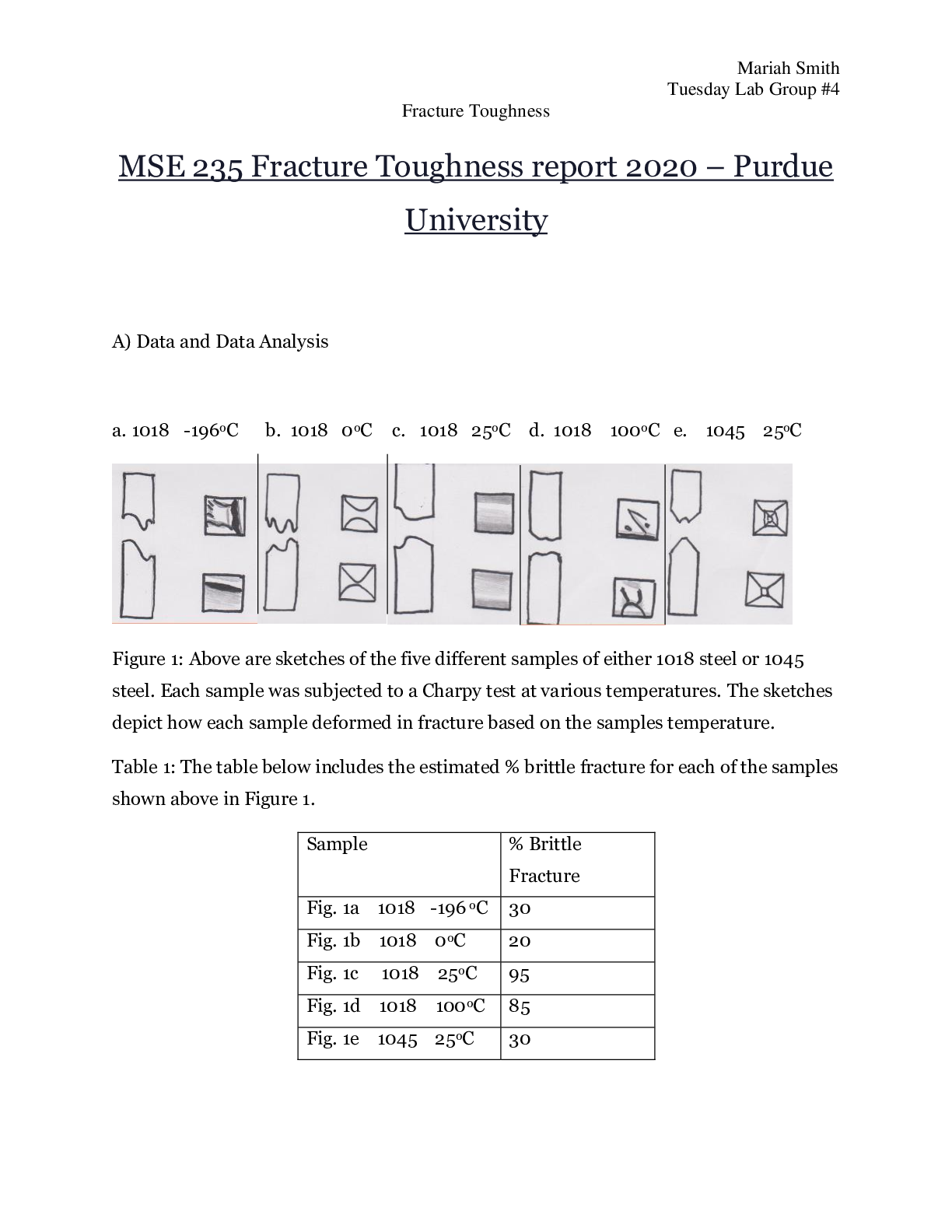
MSE 235 XRD of Metals and Crystal structure Lab template F15 2020 – Purdue University Instructions: Modify this document to report your results from the XRD of metals and crystal structures lab a ... ctivities. You will then submit this document through Blackboard using the appropriate assignment link. Your lab report will be due by 1:30 pm before your lab session during Week 4 for Group 1 & 2 or during Week 6 for Group 3 & 4.There are separate assignment links for the Tuesday lab sessions and the Thursday lab sessions – please ensure that you use the correct link to submit your lab report! Crystal Structures and X-Ray Diffraction Laboratories: comprehensive questions and report format For the format of your combined report, please neatly organize your work in the order: Section A : X-ray Diffraction Lab Section B: Crystal Structures Lab Section C: Questions based on both labs : Section A MSE235 XRD of Metals Lab Template Name: Mariah Smith Date: 9-28-2015 Group/Day: Tuesday Group 4 I. Use of the Powder Diffraction File with Chemical Information 1 • Label the diffraction peaks corresponding to each phase. o Copper => o Zinc=> • Do you think the mixture contains a greater percentage of Cu or Zn powder? Why? o The mixture contains a greater percentage of Cu powder than Zn powder because the largest Zn peak is still smaller than the smallest Cu peak 2 • Calculate the lattice parameter for an 60Cu-40Ni (at.%) solid solution. asol = .6aCu +.4aNi asol = 0.35786 • Calculate the 2 shift for the {111} peak of the solid solution when compared to that of pure FCC copper. d= 0.35786/sqrt(3) d= 0.2066 0.154 =2(0.2066)sin(theta) 2theta=43.8* Actual 2theta = 43.3* 2theta shift = 0.5* 3 • Using the atomic radii for Cu and Zn from the front cover of your textbook, estimate the peak shift for an 70Cu-30Zn (at%) FCC substitution solid solution when compared to that of pure FCC copper asol = .7aCu +.3aZn asol = 0.3661 d= 0.3661/sqrt(3) d= 0.2114 0.154 =2(0.2114)sin(theta) - - - - - - - - -- - - - - - -- - - - - - - -- - - - - - Question 3: Indium has a body centered tetragonal structure with a=0.3252nm and c=0.49461nm. The corresponding powder XRD pattern, using monochromatic radiation of wavelength of 0.1542 nm, is shown below. For the first 5 peaks of the XRD pattern, please give the multiplicity (number of equivalent planes) and list the corresponding equivalent planes. For example, for the first peak, give the multiplicity of {101} for the tetragonal structure and the equivalent planes. • {101} – Multiplicity = 8 _ _ _ _ _ _ _ _ o (101), (101), (101), (101), (011), (011), (011), (011) • {002} – Multiplicity = 2 _ o (002), (002) • {110} – Multiplicity = 4 _ _ _ _ o (110), (110), (110), (110) • {112} – Multiplicity = 7 _ _ _ _ _ _ _ _ _ _ _ o (112), (112), (112), (112), (112), (112), (112) • {200} – Multiplicity = 4 _ _ o (200), (200), (020), (020) [Show More]
Last updated: 3 years ago
Preview 1 out of 18 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 22, 2020
Number of pages
18
Written in
All
Additional information
This document has been written for:
Uploaded
Nov 22, 2020
Downloads
0
Views
156





.png)





.png)



