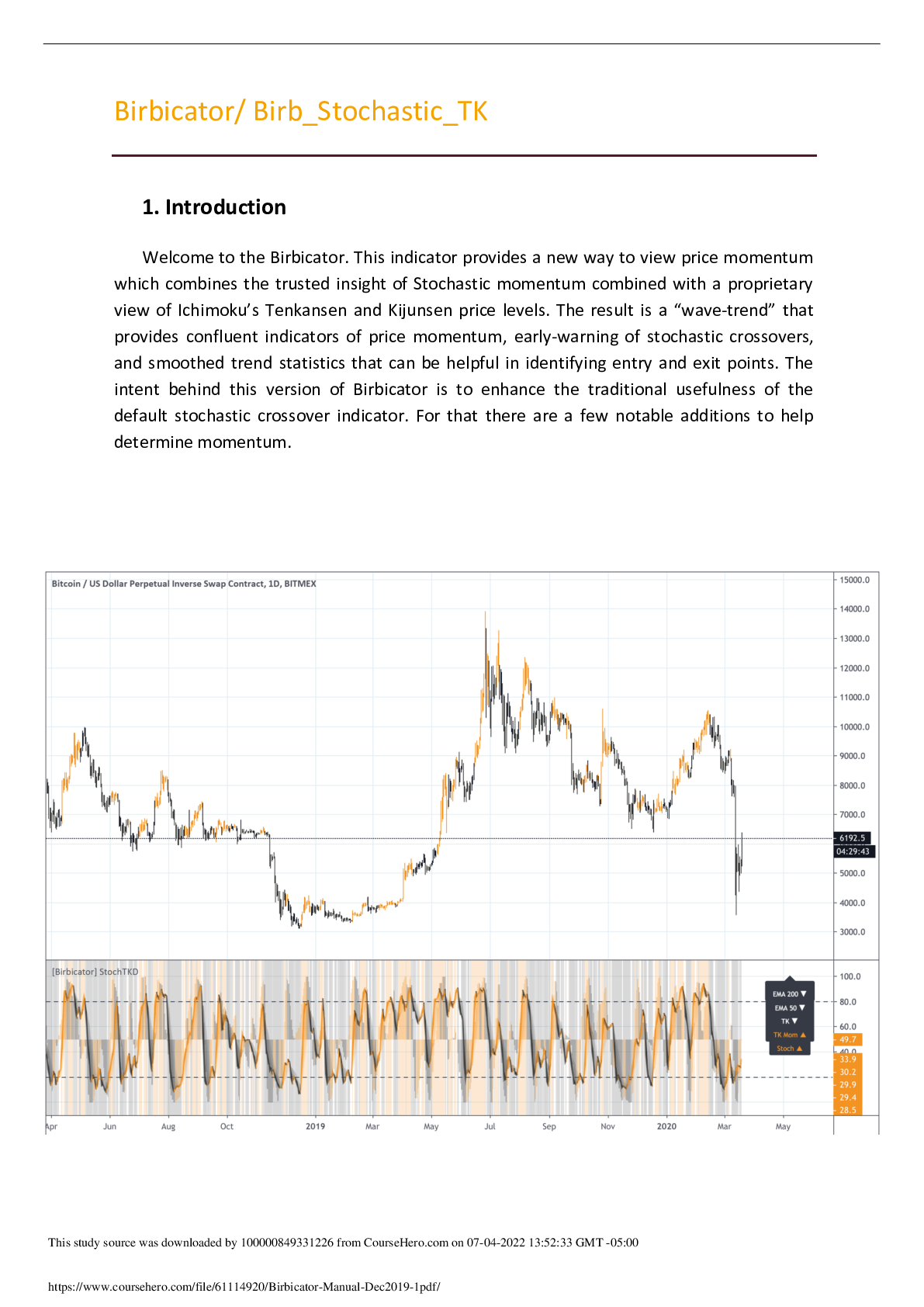
Research Paper > Molecular Structure Lab Report: Determining Polarity
Molecular Structure Lab Report: Determining Polarity
Molecular Structure Lab Report: Determining Polarity
Instructions: For this investigative ph
...
Research Paper > Molecular Structure Lab Report: Determining Polarity
Molecular Structure Lab Report: Determining Polarity
Molecular Structure Lab Report: Determining Polarity
Instructions: For this investigative phenomenon, you will investigate why certain
substances, such as oil and vinegar, don't mix. To do so, you will combine various
compounds, compare their solubility, and determine their polarity. Fill in each section of
this lab report and submit it to your instructor for grading.
Title: Determining Polarity
Objective(s): To investigate why certain substances such as, oil and vinegar, will
not mix together.
Hypothesis: If the polarity of 2 compounds are the same, then they will dissolve,
because a polar molecule added to another polar molecule will remain with the
same polarity.
Procedures:
This lab already includes materials and a summary of steps to follow. List and explain
your controlled variables, independent variable, and the dependent variable for this lab.
Materials
• deionized (distilled) water
• rubbing alcohol
• vegetable oil
• iodine
• sodium chloride (salt)
• acetic acid (vinegar)
• test tubes or clear plastic cups
• tablespoon and teaspoon
• stirring sticks
• permanent marker for labeling
Safety
· Always wear eye protection and use gloves when handling chemicals in a laboratory
area.
· Students should wash their hands thoroughly before leaving the laboratory area.
· Dispose of any chemicals by washing used test tubes with soap and water or washing
used cups then throwing them away in a trash bin.
Variables:
Remember, controlled variables are factors that remain the same throughout the
experiment. An independent (test) variable changes so that the experimenter can
see the effect on other variables. The dependent (outcome) variable will change
in response to the test variable.
Controlled variables: Solute substances such as vinegar, iodine,
vegetable oil, water, rubbing alcohol, and salt
Independent variable: Dissociation or dissolvement of molecules within
the solute substance
Dependent variable: Solvent or electronegativity of the solvent
[Show More]