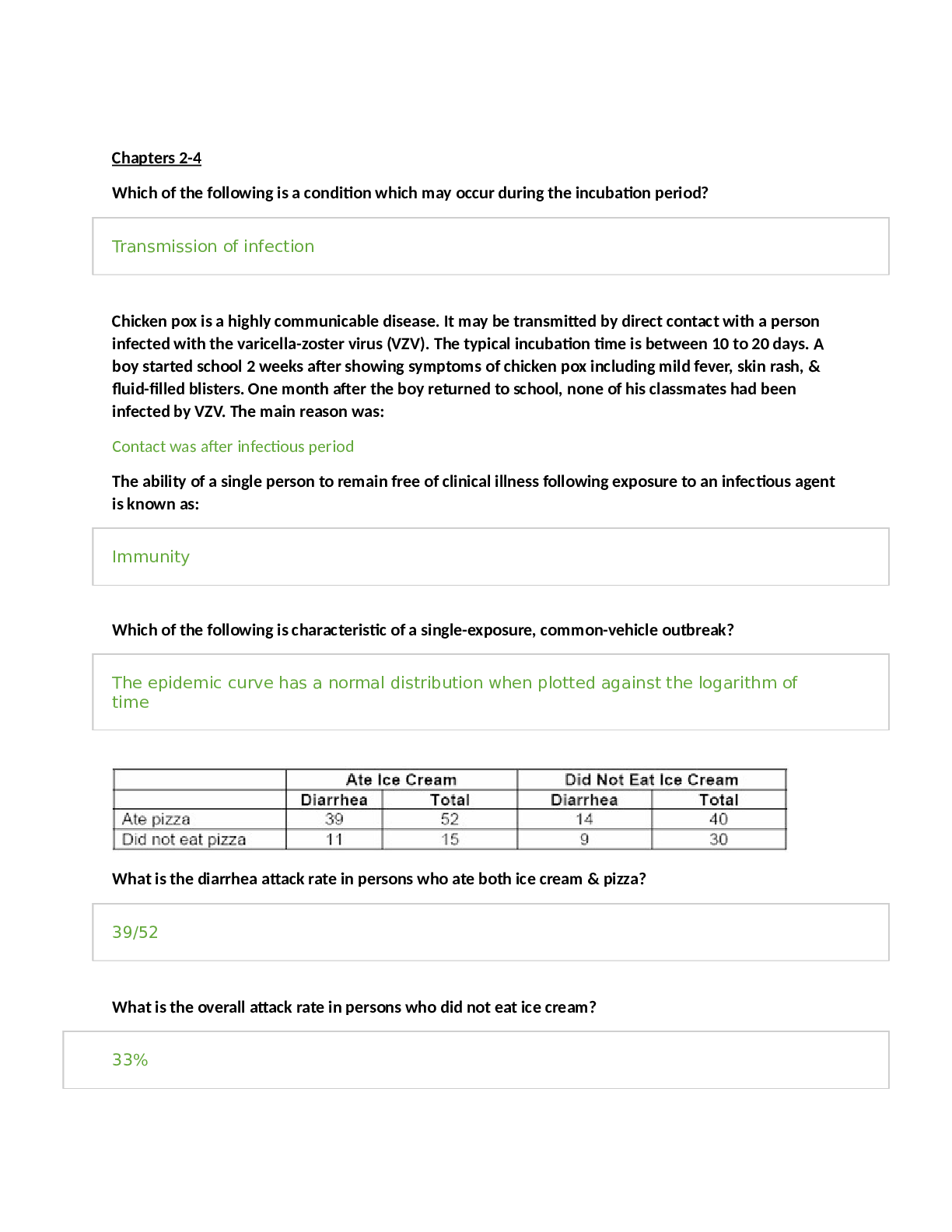
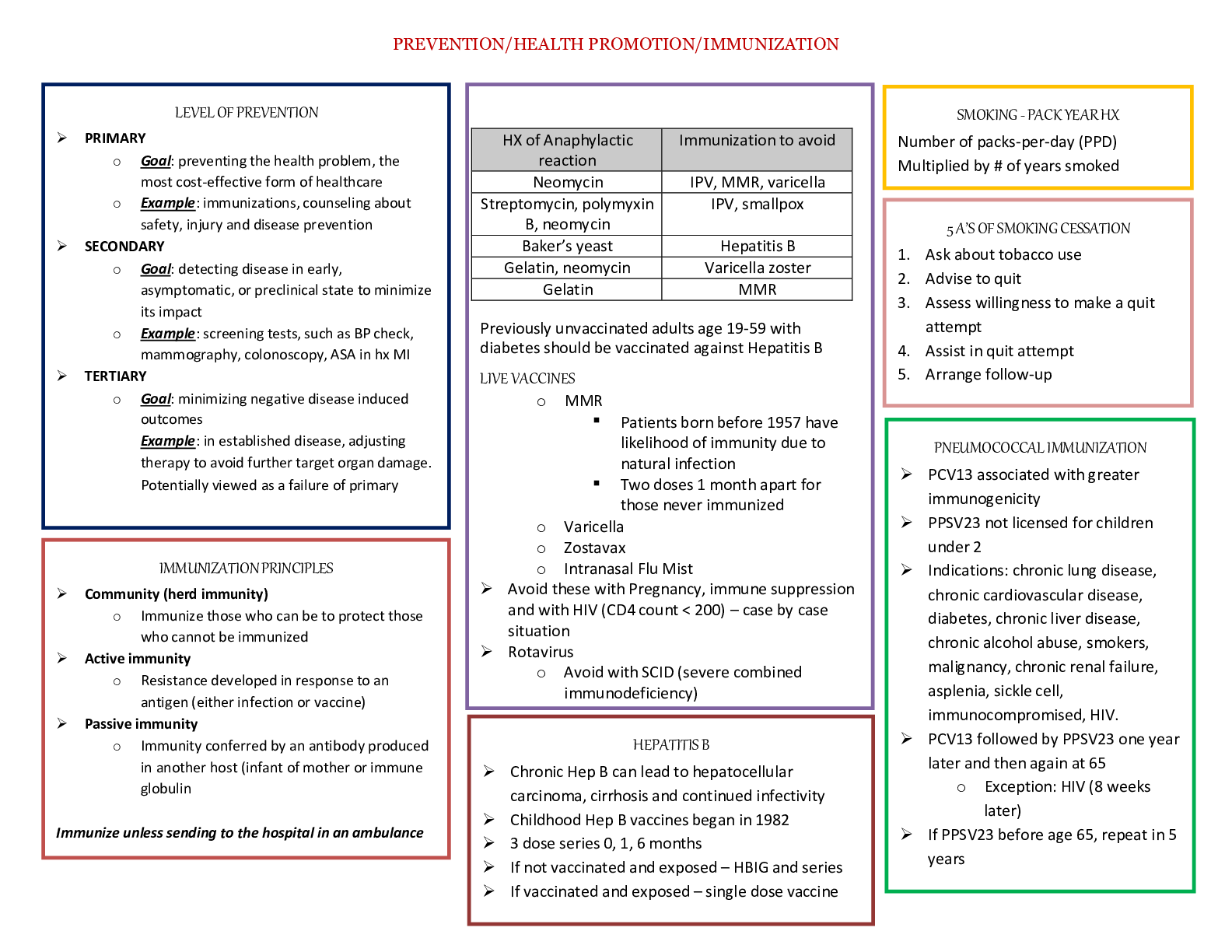
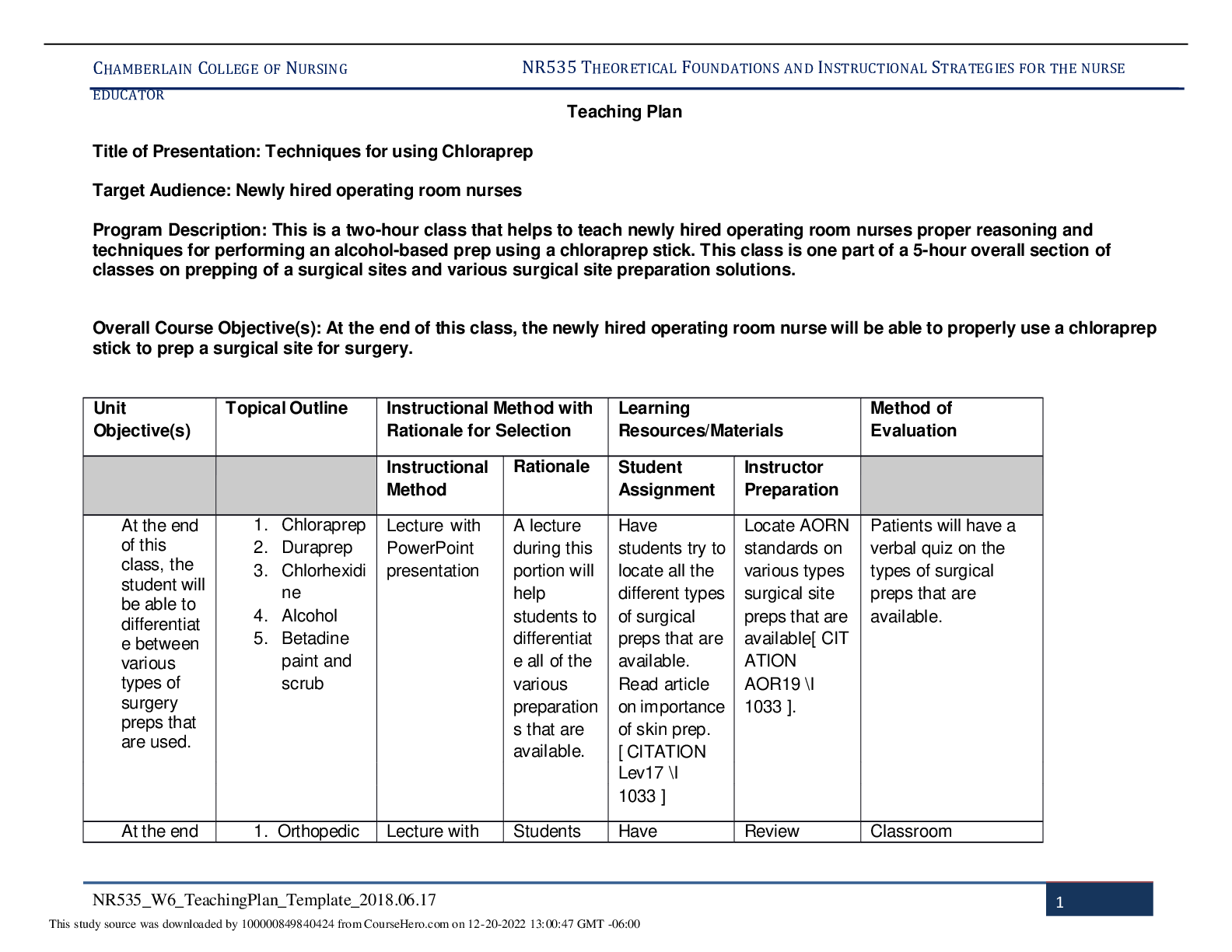
Nutrition > STUDY GUIDE > FSHN 101 Final Exam Study Guide | DOWNLOAD TO SCORE AN A (All)
FSHN 101 Final Exam Study Guide | DOWNLOAD TO SCORE AN A
Document Content and Description Below
FSHN 101 Final Exam Study Guide Section 1: Nutrition and Health 1. Intro 2. Food: Evolution Through the Ages I. The evolution of the food system in society A. Obtaining food is a fundamen ... tal activity of human life B. Early humans obtained food by hunting wild animals and gathering the edible parts of plants 1. Hunters and gatherers typically lived in egalitarian (i.e., there were no social status distinctions) bands of 20-50 people 2. They spent an estimated 20 to 30 hours per week obtaining food 3. They were nomadic, moving in seasonal rounds to exploit different foods as they became abundant C. Agricultural practices began to be developed by humans (estimated historical times) 1. Allowed humans to gain control of the food supply 2. Agriculturalists constructed towns and cities 3. Less time per person was required to grow food than to hunt and gather food, thus more free time enabled industries to develop, including the food industry 4. Overtime, the food supply improved in terms of quantity, quality, and distribution D. Food is an essential component in every society 1. Food for sustenance (concept of Food Security) 2. Additionally, food represents strong ethnic, psychological, and emotional importance 3. Food was and still is used as: gifts, rewards, punishment, a political tool, and a weapon of war II. A century of food: Shaping influences during the 20th century Poop A. Rural to Urban population shift 1. In the late 1800's, about 80% of Americans lived in rural dwellings, whereas currently, less than 2% live on farms B. A dramatic increase in crop yields and livestock production 1. Farm efficiency as measured by the farmer : consumer ratio In 1940 there was a 1: 12 ration of consumers being fed, now in 2006 there is a 1: 129 ratio. 2. Major agricultural advances which have increased the food supply (fertilizers, pesticides, herbicides, mechanization of farming, advances in veterinary medicine, breeding and genetic improvements, and biotechnology) C. Consumption of home-cooked foods shifts to processed foods and eating outside the home 1. In the late 1800's, Americans purchased commodities and processed them at home, whereas currently, Americans consumed primarily processed foods 2. Throughout the last century, we saw the development of large food companies, brand name foods, food processing techniques, and grocery stores 3. Americans currently spend about 49% of their food dollar outside of the home D. The Influence of the media 1. Cookbooks (Fanny Farmer Cookbook, 1896), Magazines, Television, Radio, etc. 2. Food is receiving more media and public attention than ever before because Americans have become more food-, diet-, health-, and safety-conscious E. Effects of World Wars I and II 1. Women enter the work force "en masse" 2. Development of new time-saving kitchen appliances 3. Major advances in all areas of science and technology, including food science F. Transportation revolution 1. Major advances in moving food from the farm to the table: boat, train, truck, and airplane 2. Increased ability to distribute food, which created the need to store and retail food 3. Food has become a global commodity 4. Automobiles and the birth of fast food restaurants G. Definition of Food Expands 1. Fair foods and Fun foods 2. Organic foods 3. Ethnic foods 4. Fine dining 5. Functional foods and dietary supplements 6. Food as entertainment III. Today's food industry in America A. We rely on a complex food system for our food supply 1. Our food supply is safer, more nutritious, more appealing, more plentiful, and more varied than ever before 2. But, the food supply is no longer in our hands alone B. The food industry is the largest manufacturing sector in the U.S. (and still growing)! 1. 1 of 7 workers in the U.S. is employed in the food processing and distribution system C. Americans spend a smaller percentage of their income on food than the citizens of any other county United States expenditure is the lowest on food, around 7.2% D. Today's food industry is consumer-driven, as opposed to research-driven (or technology-driven) E. Major priorities of today's food industry 1. Taste 2. Nutrition and Health 3. Convenience 4. Price 5. Food Safety 6. Technological innovation 7. Minimize adverse environmental impact The founding of the University of Illinois is tied to Agriculture 1.1850 Jonathan Turner promoted the idea of "industrial universities" to provide higher learning to children of the working classes 2.1862 Turner's vision was fulfilled when the Morrill Act created a system of "land grant universities" dedicated to agricultural education and research 3. 1867 The University of Illinois (then called Illinois Industrial University) was created in Champaign County for the purpose of agricultural education and research 3. Nutritional Adequacy and The Body I. Nutritional adequacy and the body Question: Does our diet affect our health status? Answer: There is no simple explanation for the relationship between diet and health. However, differences in genetic predisposition to disease can be influenced by diet, but not eliminated. II. Characteristics of nutritional adequacy A. Definition of nutritional adequacy 1. A state of good health, vitality, and a sense of wellbeing 2. A healthy body can do all of the following: a. Grow b. Repair damage c. Maintain structure and functions d. Reproduce (at a specific time in the life cycle) e. Do useful work 3. Since every individual is genetically distinct, we must think of nutritional adequacy in terms of a distribution rather than a single value Average requirement for energy must be within 2 standard deviations from the average. III. States of nutritional health Question: If a little is good, then is more better? Answer: Not true for nutrient and caloric intake A. The four states of nutritional health 1. Overnutrition: dietary excesses of nutrients and calories 2. Desirable status: sufficient amount of nutrients and calories 3. Undernutrition: nutrients and calorie stores depleted 4. State of body deficiency: reduced biochemical function and clinical symptoms IV. Consuming a balanced diet A. Description 1. A diet that provides: a. the necessary levels of essential nutrients, and b. adequate energy in the form of calories 2. How a balanced diet is determined, however, is complex B. Dietary Reference Intakes 1. Definition: Dietary Reference Intakes are reference values that are quantitative estimates of nutrient intakes to be used for planning and assessing diets for healthy people. They include RDA values and three other types of reference values, EAR, AI, and UL 2. The DRIs greatly expand and replace the previous RDA series (1943-1989) and are for both Americans and Canadians 3. Revisions were done by panels (organized by groups of nutrients) and 2 subcommittees 4. The nutrient groups: a. Dietary Reference Intakes for Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride ; published 1997 b. Dietary Reference Intakes for Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; published 2000 c. Dietary Reference Intakes for Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; published 2000 d. Dietary Reference Intakes for Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; published 2001 e. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; published 2002 f. Dietary Reference Intakes for Electrolytes And Water ; report released Feb. 11, 2004 5. The 2 subcommittees: a. Upper Reference Levels of Nutrients b. Interpretation and Uses of the DRIs 6. The Four Dietary Reference Intake (DRI) values are: a. Estimated Average Requirement (EAR) b. Recommended Dietary Allowances (RDAs = EAR + 2SD) c. Adequate Intake (AI) Levels d. Tolerable Upper Intake Level (UL) 7. DRI Summary Tables 8. Issues still to consider: a. Values for persons with disease states? b. Values for possible reducing the risk of chronic diseases? c. Nutrient interrelationships? 4. Macronutrients: Carbs, Proteins, Lipids & Water 5. -- I. Human energy needs A. Energy is measured using the common unit of dietary energy known as the calorie (abbreviated as cal) B. Definitions 1. calorie = the quantity of heat required to raise the temperature of 1.0 g of water by 1 degree Celsius (°C) 2. 1000 calories = 1 kilocalorie (abbreviated as kcal) = 1 Calorie (abbreviated as Cal) 3. Energy from foods is given in Calories (note capital C) or kilocalories 4. 3500 kcals equals approximately one pound C. DRI values for energy 1. Estimated energy requirement (EER) represents the average needs of a population II. Measuring the gross composition and energy content of foods A. Proximate analysis 1. Water and solids by drying loss 2. Protein by nitrogen content 3. Lipid by solvent extraction 4. Minerals from ash 5. Carbohydrates (CHO) by difference B. Calculation - A technique used to estimate the total potential energy of a food sample 1. Based on known average caloric values a. Carbohydrate = 4 kcal/g b. Protein = 4 kcal/g I like poop lol c. Lipid = 9 kcal/g d. Alcohol = 7 kcal/g (Non-nutritive kcals) e. Water = 0 kcal/g Know how to calculate total kcals and percent kcals from fat III. Metabolism A. Description 1. The body operates like a complex chemical factory with a special rule: a. Reaction temperatures must be kept at 37 °C (normal body temperature) 2. Our metabolism encompasses the entire network of biochemical processes involved in maintaining life 3. Anabolic pathways build compounds, using energy input 4. Catabolic pathways break down food components into small units, yielding energy a. CHO, proteins, lipids (and alcohol) are oxidized (broken down in the presence of oxygen) to produce energy (in the form of ATP energy), water, and carbon dioxide B. Energy Balance 1. Definition - The amount of energy you put into your body (food calories) versus the amount of energy you expend. 2. Energy expenditure a. Basal Metabolic Rate (or Basal Energy Expenditure): Most of the body's energy, about 60-70%, goes to supporting the ongoing metabolic work of the body's cells. This includes such activities as heart beat, respiration and maintaining body temperature. b. Physical Activity: Varies widely within a population (from sedentary to very active) and contributes 20-30% to the body's total energy output (Physical Activity Table) c. Diet-induced Thermogenesis [DIT] (also referred to as Thermic Effect of Food): The last component to calculate has to do with your body's management of food. The increase in energy required to digest food is referred to as the thermic effect of food (TEF) and contributes 10% to the body's total energy expenditure and is different for each macronutrient based on the amount of ATP required for the initial steps of metabolism and storage (protein > CHO > lipid). 3. Three possible energy balance situations Weight gain (food, CHOs, alcohol, protein, lipids), weight maintenance, weight loss (DIT [diet induced thermogenesis], physical activity, basal expenditure) a. Weight Maintenance: Energy intake (food calories) = Energy expenditure [Neutral energy balance] b. Weight gain: Energy intake (food calories) > Energy expenditure [Positive energy balance] c. Weight loss: Energy intake (food calories) < Energy expenditure [Negative energy balance] IV. Macronutrients: Nutritional Characteristics A. Carbohydrates 1. Are classified nutritionally into three categories a. Simple sugars (digestible CHO) b. Complex CHO (digestible CHO) c. Fiber (nondigestible CHO) 2. Primary role of digestible CHO's is to provide energy to cells in the body, especially the brain, which is the only CHO-dependent (glucose) organ in the body; primary role of non-digestible CHO is fecal bulk and laxation 3. Glucose can be produced by the body, however, consumption of digestible CHO's facilitate efficient use of other nutrients and energy a. Example: protein sparing effect b. Example: efficient lipid oxidation 4. Digestible CHO RDA set at the minimum amount of glucose required by the brain without depending on fat or protein as an alternative energy source; nondigestible CHO (called Total Fiber) have an AI 5. Contribution to daily caloric intake for digestible CHO a. DRI Acceptable Macronutrient Distribution Range (Adults >18 yrs): 45-65% of energy b. Current USDA recommended consumption level: 55% of energy c. Observed U.S. consumption level: 50% of energy 6. The most inexpensive source of food energy 7. Dietary sources of digestible CHO and nondigestible CHO a. Examples: 8. Over the last century, humans have consumed more simple sugars (and alternative sweeteners) and less complex CHOs and fiber B. Proteins 1. Are composed of 20 amino acids a. 9 are indispensable for humans These are: Phenylalanine, Valine, Threonine, Tryptophan, Isoleucine, Methionine, Histidine, Leucine, and Lysine 2. Protein is essential in the diet (quantity and quality) 3. Protein quality depends on the amino acid composition a. Example: whole egg vs. gelatin 4. RDA values for protein and 9 indispensable amino acids 5. Contribution to daily caloric intake a. DRI Acceptable Macronutrient Distribution Range (Adults >18 yrs): 10-35% of energy b. Current USDA recommended consumption level: 15% of energy c. Observed consumption level: 16% of energy 6. The most expensive source of food energy 7. Dietary sources a. Examples: 8. Protein malnutrition is called kwashiorkor C. Lipids 1. Are classified into four categories a. Free fatty acids (or "FFA"): may be saturated, monounsaturated and polyunsaturated b. Triglycerides: glycerol + 3 fatty acids c. Phospholipids: contains phosphorus and fatty acids d. Sterols: multi-ring structures (e.g., cholesterol) 2. Essential Fatty Acids a. Linoleic acid (18C, 2 double bonds, [omega] - 6) b. Linolenic acid (18C, 3 double bonds, [omega] - 3) 3. AI for Linoleic acid and Linolenic acid (Note: No AI or RDA for Fat for Children or Adults) 4. Contribution to daily caloric intake a. DRI Acceptable Macronutrient Distribution Range (Adults >18 yrs): 20-35% of energy b. USDA recommended consumption level: no more than 30%, with approximately 10% each from saturated, monounsaturated and polyunsaturated fats c. Observed consumption level: 34% of energy 5. Other recommendations a. Food and Nutrition Board recommends that cholesterol, trans fatty acids, and saturated fatty acids intake be "as low as possible," while consuming a nutritionally adequate diet b. American Heart Association recommends no more than 300 mg of dietary cholesterol/day 6. Dietary sources a. Examples: 7. Other functions in food systems: carriers of lipid-soluble vitamins, provides satiety, flavor and texture D. Water 1. Is classified as a polar compound 2. Is indispensable for life a. Constitutes about 50% to 70% of the human body (by weight) b. Maintains homeostasis in the body and allows for transport of nutrients to cells and removal and excretion of waste products of metabolism c. Thirst occurs at about 1% water loss (based on initial body weight) due to dehydration; death occurs at 20% loss d. We can live about 8 weeks without food, but only a few days without water 3. AI for water includes all water contained in food, beverages, and drinking water 4. No contribution to daily caloric intake 5. Dietary sources a. Example: 6. Dramatically effects the chemical, microbial, and textural stability of foods 6. Micronutrients: Vitamins & Minerals I. Metabolism of micronutrients A. Factors that influence the amounts of micronutrients needed by the body 1. Life Stage Group (age and gender) 2. Physiological status (e.g., pregnancy, lactation, health status) 3. Lifestyle (e.g., activity level, stress) B. Factors that influence the absorption or bioavailability of ingested micronutrients 1. Source of the vitamins and minerals a. Food source Example: Calcium b. Chemically synthesized vitamins 2. Chemical state of the vitamins and minerals a. Example: Heme-iron versus Nonheme-iron 3. Interrelationships between ingested micronutrients a. Inhibit example: Iron and Zinc b. Promote example: Calcium and Vitamin D II. Vitamins A. Definition, history, and classification 1. Carbon-containing compounds required in very small amounts (milligram [mg = 10-3 g] to microgram [µg, = 10-6 g]) by the body to promote and regulate chemical reactions and processes 2. Casimir Funk (1884-1967) discovered the first vitamin (Vitamin B) in 1911 and published the text The Vitamines in 1922 3. Are divided into 2 classes a. Water-soluble b. Fat-soluble B. Optimal consumption range 1. Dose-health response concept 2. How long before deficiency symptoms begin (stores exhausted)? a. Varies depending on the vitamin (e.g., Thiamin ~10 days; Vitamin C ~20 to 40 days) 3. Consequences of too little or too much a. Example: Vitamin A C. Fact or fiction? 1. "Vitamins give you pep and energy" 2. "Organic or natural vitamins are nutritionally superior to synthetic vitamins" 3. "Even if you consume a well balanced diet you cannot get enough vitamins from the foods you eat" 4. "Vitamin C prevents the common cold" 5. "The amount of vitamins that I consume is directly proportional to my health status" 6. "Vitamin supplements protect my body against harmful chemicals and pollution better than vitamins from foods" III. Minerals (or Elements) A. Definition and classification 1. The fundamental chemical elements that are used in the body to help form cellular and tissue structures and regulate metabolic processes 2. Are divided into 3 classes a. Major b. Trace c. Ultratrace (may be of benefit to humans) 7. Nutrients: Digestion and Absorption I. The digestive process A. Summary 1. The food we consume is broken down into usable nutrient forms 2.The nutrients are absorbed into the bloodstream and distributed to the cells in the body a. This process is accomplished and regulated by the: 1) Gastrointestinal (GI) tract 2) Accessory digestive organs (salivary glands, pancreas, liver, and gallbladder) 3) Hormonal system 4) Nervous system 3.Waste is eliminated II. Food breakdown and absorption A. Functions of the mouth 1.Chewing action a. Facilitates easy swallowing by reducing the particle size of food b. Increases the surface area of food to enhance enzymatic attack 2.Saliva production by the salivary glands a. Lubrication b. Enzymes break down large carbohydrates 3.Digestion actually begins even before we consume foods B. Functions of the esophagus 1.Transports chewed food particles and saliva to the stomach C. Functions of the stomach 1. Volume (about 4 cups), residence time (2 to 4 hours), and pH < 2 2.Physically breaks down food by a. Grinding b. Mixing 3.Produces chyme a. Contains food, digestive acids, and enzymes b. Heartburn (esophagus) 4.Initiates protein digestion into amino acids by using pepsin 5.Absorbs ~ 20% of consumed alcohol, some H2O, and some lipids D. Functions of the small intestine 1. Length (about 10 feet), width (1 inch), and residence time (3 to 10 hours) 2.Prepares and breaks down food chemically using the following reagents: a. Sodium bicarbonate: pancreas; neutralizes acid 1) Cause of some ulcers: Bacterial infection (Helicobacter pylori) b. Bile: produced in liver; stored in gallbladder; decreases fat globule size and suspends the fat in the watery chyme mixture c. Enzymes: intestinal wall and pancreas; breaks down food into usable forms 3. Transports the mixture of food and digestive juices by muscle contractions 4. Absorbs nutrients and fluids a. About 95% of consumed nutrients (solids) are absorbed 1) Passive absorption 2) Facilitated passive absorption (carrier protein required, but no energy) 3) Active absorption (carrier protein and energy required) 4) Phagocytosis b. About 85 - 90% of consumed fluids are absorbed 5.Transports waste to large intestine E. Functions of the large intestine 1.Length (about 3.5 feet) and residence time (24 up to 72 hours) 2.Absorbs some H2O and minerals 3.Digests some residual plant fibers by bacterial fermentation 4.Prepares waste for elimination III. Distribution of usable nutrient forms A. Carbohydrates (CHOs) 1. Broken down to monosaccharides 2.Transported to liver via the portal vein 3.Metabolic destinations a. Transformed into glucose and released to bloodstream b. Processed into glycogen c. Processed into fat and stored (least likely) B. Proteins 1. Broken down into amino acids 2.Transported to liver via the portal vein 3.Metabolic destinations a. Used by the body b. Converted into usable forms of energy c. Processed into fat and stored C. Lipids 1. Broken down to fatty acids and monoglycerides 2. The carbon chain length affects their fate after absorption: a. Short and medium chain fatty acids (less than 12 carbon atoms) are transported to the liver via the portal vein b. Long chain fatty acids (12 or more carbon atoms) are: 1) Re-formed into triglycerides t 2) Triglycerides aggregate and are combined with cholesterol, protein, and phospholipids to form chylomicrons (also called lipoproteins) and enter the lymphatic system 3) Eventually the chylomicrons enter the bloodstream, and 4) Once in the bloodstream, the triglycerides are broken down into fatty acids and glycerol by an enzyme in the blood vessels 3. Metabolic destinations a. Used by the body for energy and other functions b. Stored as adipose tissue 8. Dietary Guidelines for Americans and the Food Guide Pyramid I. Government publications providing summary dietary recommendations and information A. Purposes of these documents 1. To help Americans put into practice the DRI information 2. To help answer questions related to diet and health, such as: a. What foods should Americans eat to achieve good health? b. How much should Americans eat to achieve good health? c. What role does physical activity play in helping Americans achieve good health? II. 2010 Dietary Guidelines for Americans, 7th edition (released January 31, 2011) A. Developed by 1.U.S. Department of Agriculture (USDA) 2.U.S. Department of Health and Human Services (DHHS) 3.Based on recommendations of the Dietary Guidelines Advisory Committee B. Target audience 1. Oriented toward policy-makers, nutrition educators, nutritionist, and healthcare providers (rather than to the general public) 2. Consumer brochure entitled "Dietary guidelines – selected messages for consumers" based on the Dietary Guidelines for Americans is provided for the general public C. Target population 1. Healthy children (ages 2 years and older) and adults of any age D. Two principle themes 1. Maintain calorie balance over time to achieve and sustain a healthy weight 2. Focus on consuming nutrient-dense foods and beverages E. Four inter-related focus areas, with general key recommendations and key recommendations for specific population groups in each focus area (see DGA Executive Summary and Consumer Brochure PDF handouts) Focus area 1: Balancing calories to manage weight Focus area 2: Foods and food components to reduce Focus area 3: Foods and nutrients to increase Focus area 4: Building healthy eating patterns III. The 2011Choose MyPlate initiative called MyPlate A. History 1.Mid-1950s: Four Food Groups a. Milk b. Meat c. Fruits and vegetables d. Breads and cereals 2.1979: USDA revised the groups into the "Hassle-Free Daily Food Guide" and added a 5th group e. Fats, sweets, and alcoholic beverages 3. August 1992: The Food Guide Pyramid first published (updated in 1996) 4. April 2005:MyPyramid replaces the 1992/1996 Food Guide Pyramid 5. June 2011: MyPlate replaces the 2005 Food Guide Pyramid B. 2011 MyPlate developed based on the 2010 Dietary Guidelines for Americans 1. Target population a. Same as 2010 Dietary Guidelines (2 years of age and older) 2. How to use MyPlate a. Getting Started with MyPlate b. Daily Food Plan 9. Dietary Assessment: Nutrition Facts Label I. The Nutrition Facts Label A. The Nutrition Labeling and Education Act (NLEA) of 1990 1. Increased government regulation of food labels a. Regulations announced on Wednesday, 2 December 1992 and published in the Federal Register on Wednesday, 6 January 1993 b. New labels were required on food packages as of August 1994 2. Mandated nutritional labeling for most foods 3. Established standardized serving sizes within product categories 4. Set definitions for uniform use of nutrient content claims on labels a. Claims can describe the level of a nutrient or dietary substance in the product using terms such as "free," "high," and "low" OR b. Claims can compare the level of a nutrient in a food to that of another food (called Relative (or Comparative) Claims) using terms such as "more," "reduced," and "lite" 5. Set criteria for approval (by FDA) and use of health claims on labels a. Health claim is defined by the Act as: "Any claim that expressly, or by implication... characterizes the relationship of any substance to a disease or health-related condition." b. Currently allowed health claims on food package labels B. Daily Values (DV's): The Standard Used for Food Labeling 1. It is not possible to use all of the age and gender specific DRI values on the food label 2. The Food and Drug Administration (FDA) has established a set of generic standards to be used, called Daily Values 3. The Daily Values are based on two sets of dietary standards: a. Reference Daily Intakes (RDI's) for vitamins and minerals 1. Currently based on the 1968 RDA's 2. Uses the highest RDA value of any age/gender category b. Daily Reference Values (DRV's)for other dietary components, such as total fat, saturated fat, cholesterol, sodium, total carbohydrates, and dietary fiber (Note: Usually no protein value given) c. To decrease confusion the terms Reference Daily Intakes and Daily Reference Values do not appear on the label 4. Nutrient content on the Nutrition Facts label is expressed as a percentage of the Daily Value (%DV) a. Mandatory and optional food components b. Exceptions: If a claim is made about any of the optional components, or a food is fortified or enriched with any of them, nutrition information for the specific component also becomes mandatory C. Reading the label: What information is available? 1. Realistic, standardized serving size for similar products 2. Number of total Calories and Calories from fat per serving 3. Daily Value standards for a 2000 Calories and 2500 Calories diets 4. % Daily Values based on 2000 Calories diet 5. List of ingredients in descending order by weight Section 2: Food Composition and Chemistry 1. Food Chemistry- The Basics I. Exploring the relationship between food and chemistry Question: What does chemistry have to do with food? Answer: Everything! A. Some questions that can be answered by studying the chemistry of food materials: 1. Why does sucrose dissolve in water and taste sweet? 2. Why does the crust of bread become brown during baking? 3. What happens to eggs and meat during cooking? 4. Why don't water and oil separate in salad dressings? 5. Why is butter semi-solid and vegetable oil liquid at room temperature? B. Functionality of the macronutrients 1. In addition to providing nutritional value, macronutrients contribute important chemical and physical properties (collectively known as functionality) to foods 2. To understand the functionality of the macronutrients we need to understand their chemistry II. Review of chemistry A. Fundamental concepts and definitions 1. All matter is composed of atoms or elements 2. The known elements have been arranged in a Periodic Table where all elements within a given column have similar chemical properties 3. Definitions of elements, compounds, and mixtures a. Elements (1) Composed of only one kind of atom (2) Atoms are composed of subatomic particles: protons, neutrons, and electrons (3) Each element has a fixed number of protons and electrons, but the number of neutrons can vary (resulting in isotopes) 4) Electrons are important in forming chemical bonds b. Compounds (or Molecules) (1) Composed of two or more elements (2) Simplest form is a binary compound Examples: (3) To form compounds, elements are held together by net electrical forces of attraction called chemical bonds a. a chemical bond forms between elements if the resulting arrangement of elements has a lower energy than the sum of the energies of the separate elements c. Mixtures (1) Composed of two or more compounds (2) Compounds retain their discrete chemical identity in the mixture Examples: III. Types of chemical bonds and intermolecular forces A. Chemical bonds between elements a. A few rules to remember (1) The Aufbau principle: every element has the same number [and placement] of electrons as the one before it, plus one extra (2) The Octet Rule: elements tend to want to gain or lose electrons to attain the same electron configuration as the nearest noble gas 1. Ionic bonds: attraction between opposite charges of cations and anions a. Complete transfer of one or more electrons from one element to another followed by an attraction of these oppositely charged ions (1) Example: sodium chloride (NaCl) 2. Covalent bonds: bonded elements (or atoms) share pairs of electrons a. Examples: methane (CH4) and water (H2O) b. Single, double, or triple bonds are possible depending on how many pairs of electrons are shared between the elements B. Intermolecular forces between compounds 1. London Dispersion Forces: attractive forces that arises from interaction between transient instantaneous electric dipoles on neighboring molecules; common to all compounds 2. Dipole-dipole interactions: attractive forces between two permanent electric dipoles; common to polar molecules, in addition to London forces 3. Hydrogen bonds: consists of a hydrogen atom lying between two strongly electronegative atoms and covalently bonded to one of them (O, N, or F) a. The electronegative atoms may be located on different molecules or in different regions of the same molecule b. Examples: H2O and proteins IV. Phases (or "states") of matter A. Three fundamental phases 1. Solid: characterized by a fixed volume and a fixed shape 2. Liquid: characterized by a fixed volume and the absence of a fixed shape 3. Gas: characterized by the absence of both a fixed volume and a fixed shape 4. Foods can be composed of one, two, or all three states Examples: B. One pseudo-phase (or pseudo-state) 1. Supercritical fluid C. Variables that determine the phase of matter 1. Type of material 2. Pressure 3. Temperature D. Phase diagrams 1. A plot of pressure vs. temperature for a specific material 2. A general phase diagram for a pure compound a. "TP" is the triple point, where all three phases (solid, liquid, and gas) are in equilibrium b. "CP" is the critical point, the beginning of the supercritical fluid region (a range of very high temperature and pressure values) Example: c. Along the curve A-TP the solid and gas phases are in equilibrium; along the curve TP-CP the liquid and gas are in equilibrium, and along the curve TP-B the solid and liquid are in equilibrium d. Effects of temperature and pressure on molecule mobility V. Chemical formulas and structures A. Formula 1. Signifies which elements and how many atoms of each are present a. Example: Glucose - C6O6H12 B. Structure 1. Exhibits which atoms are chemically bound to each other 2. Exhibits the spatial arrangement of the atoms a. Example: Glucose VI. Functional groups A. Role in organic chemistry 1. Functional groups define an organic family of compounds 2. The structure of a group is related to its physical and chemical or "physico-chemical" function (called a structure-function relationship) 3. Some functional groups important in food systems are given in Table 11.2 Examples: Alcohols, ethers, disulfides, aldehydes, ketones, carboxylic acids, esters, amines (1, 2, and 3), and amides 2. Water. It’s Role in Life and In Foods I. Water is indispensable for life! A. All living beings require water to survive B. Water is one of the most abundant and unique chemicals on earth C. All foods contain at least some water II. Chemical and physical properties of water A. Composition and structure 1. Water is composed of one oxygen atom and two hydrogen atoms joined by polar covalent bonds a. Because oxygen is an electronegative element, there is a partial positive charge on each of the hydrogen atoms and a partial negative charge on the oxygen atom b. Note that the oxygen atom contains two lone-pair orbitals 2. With other polar compounds C. Mobility of water 1. The water molecule has three types of motions: D. Phases (or states) of water 1. Solid (ice), Liquid, Vapor (gas), and Supercritical fluid 2. Phase diagram for pure water a. Names of the 6 phase transitions: 1) melting 2)freeezing 3)vaporization 4)deposition 5)condensation 6) b. "TP" is the triple point, where all three phases (solid, liquid, and gas) are in equilibrium. "TP" for water is: c. "CP" is the critical point, the beginning of the supercritical fluid region (a range of very high temperature and pressure values). "CP" for water is: d. Effects of temperature and pressure on molecular mobility e. Effect of pressure on the temperature at which phase transitions occur 3. Energy requirements associated with the phase diagram of water a. Energy (cal/g) in the form of sensible heat must be added or removed from the water to change its temperature b. Energy (cal/g) in the form of latent heat must be added or removed from the water for a phase transition to occur c. For any substance (including water), no change in temperature occurs during a phase transition d. More energy must be added for a liquid to gas transition than an ice to liquid transition e. The temperature at which these phase transitions occur are dependent on pressure 4. Phase transitions are important in many food processes a. Evaporation: b. Freezing: c. Sublimation: E. Unique physical properties 1. Unusually high freezing point and boiling point 2. Unusually high specific heat (1 cal/g °C) a. Water regulates our body temperature, as well as the temperature of the earth 3. Solid state is less dense (greater volume per unit mass) than the liquid state III. Importance of water in foods A. Food quality 1. Taste (water is a solvent for taste stimulant molecules like sucrose) 2. Texture (water functions as a plasticizer) B. Food stability 1. Depends on the mobility of the water in the food a. Water mobility: the availability of the water to participate in chemical reactions and microbial growth and affect food texture is important b. In general, low water mobility results in high product stability 2. Measuring water mobility: water activity (Aw): a. Aw = Pwater in food / Ppure water b. Where Pwater in food is the partial vapor pressure of the water above the food and Ppure water is the vapor pressure of pure water at the same temperature c. Aw in food is related to % Equilibrium Relative Humidity (ERH) as Aw = % ERH/100 3. Electronic instruments can be used to measure the Aw of a food 4. Moisture content and Aw of selected food products 5. Growth of microorganisms is dependent on Aw 6. The rate of chemical reactions is also dependent on Aw a. Most reaction rates increase with increasing Aw, except b. Lipid oxidation - high reaction rate at low Aw 7. Two challenges for food scientists: a. Keeping oil and water mixed (1) Emulsifiers (movie) b. Producing shelf-stable dual textured foods (1) Concept of equal Aw 3. Carbohydrates: Simple I. Carbohydrates A. Classification and chemical groups 1. Carbohydrates (CHO) are classified by polymeric size: a. Simple CHO (or "sugars"): b. Complex CHO: 2. Contain hydroxyl (-OH), aldehyde (-HC=O) and/or ketone (-R'C=O) chemical groups II. Simple sugars A. Chemical, structural, and physical properties 1. Simple sugars are mono- and di-saccharides that are present in many different foods 3. Simple sugars are hydrophilic and readily form hydrogen bonds with water and solubilize a. Saturation solubility is dependent on sugar type and temperature b. Definition of a saturated solution: B. Functional properties 1. Provide a sweet taste a. Sugar molecules align with the sweetness receptors on the tongue to elicit a sweet taste response b. Sweet taste perception varies among the simple sugars c. Sucrose is considered the "gold standard" of sweet taste perception 2. Provide texture and structure to some foods a. Example: 3. Provide viscosity and mouthfeel ("body" of liquid foods) a. Example: 4. Decrease the Aw of food systems 5. Serve as reactants in important chemical reactions a. Maillard browning reaction (1) Occurs between a reducing sugar and an amine group that results in a series of reactions which produce brown colored compounds (a) Common reducing sugars (b) Can sucrose participate in Maillard browning? Why or why not? b. Carmelization (1) Occurs when a sugar is heated to a temperature greater than its melting point (MP, for sucrose = 320 °F [160 °C]) (2) The high temperature causes a series of reactions to polymerize the sugar molecules into colored compounds 6. Serve as fermentation substrate for microorganisms a. Example: b. Example: III. Alternative sweeteners A. Low- and No-Calorie sweeteners 1. Functional properties a. Replace the sweet taste of simple sugars b. Decrease the caloric content of foods ES = Equivalent sweetness compared to sucrose 2. Chemical composition a. Aspartame: 2 amino acids (L-phenlyalanine and Laspartic acid) b. Sucralose: chlorinated sucrose c. Neotame: 2 amino acids (L-phenlyalanine and L-aspartic acid combined with two functional groups - methyl ester and neohexyl) d. Alitame: 2 amino acids (L-aspartic acid and D-alanine) B. Reduced-Calorie sweeteners 1. Functional properties a. Replace some of the sweet taste of simple sugars b. Decrease the caloric content of foods (except trehalose) c. Other benefits as listed in Table 13.5 14. Carbohydrates: Complex I. Starch A. Function in plants and sources 1. Starch is stored by plants as an energy reserve in packets called granules 2. Main sources of starch include cereals, legumes, and some vegetables 3. Starch granules from different plants vary in size and shape, but not in composition B. Composition and structure 1. Starch molecules are composed of glucose units linked together to form polymers 2. Contains two distinct polymeric forms Four parts. Four parts. which are? C. Functionality in foods 3. Starch as an individual food ingredient a. Much of the starch used as a food ingredient comes from maize (corn) b. Starch thickens liquid and semi-liquid food systems by gelatinization: (1) (2) c. Functionality problems (1) Retrogradation: recrystallization of starch after gelatinization has occurred (a) Culminates in water expulsion from a starch gel - this phenomenon is called syneresis (2) Lack of refrigeration and freeze-thaw stability d. Starches are often chemically and/or genetically modified in order to improve their functional properties and to create new ingredients for specific food applications 4. Starch in flour a. Much of the flour used as a food ingredient comes from wheat b. Flour is one of the principle ingredients used in doughs and batters (1) Starch and protein provide structure and body in baked products (2) The extent of starch gelatinization in baked products depends on the amount and availability of water II. Dietary fiber A. Function in plants and sources 1. Fiber components are found primarily in or around the cell wall of plants, often playing an important structural role 2. Sources include the outer bran layers of cereal grains, fruits, and vegetables B. Composition, structure, and classification 1. Composed of various saccharide units some of which are linked by beta- (1-->4) bonds a. These linkages cannot be digested by human enzymes b. Thus these polysaccharides are indigestible (not broken down by digestive enzymes) 2. Classified by ability to dissolve in water a. Insoluble (not functional in foods) (1) Do not dissolve in water (2) Are not metabolized (fermented) by bacteria in the large intestine (3) Examples: cellulose and hemi-cellulose (4) Structural comparison between starch and cellulose b. Soluble (functional in foods) (1) Dissolve or swell in water (2) Are metabolized (fermented) by bacteria in the large intestine (3) Examples: pectin and gums (also called hydrocolloids) 3. We need both insoluble and soluble fibers in our diet 4. Food scientists also classify fibers by their degree of fermentability and by their level of viscosity C. Functionality of soluble fiber in foods 1. Give highly viscous solutions and/or gels at low concentrations 2. Examples of functionality in specific food systems: 5. Amino Acids: Protein Building Blocks Refer to Lecture 15 Power Point for More Detailed Notes B. Chemistry –Each amino acid contains an alpha carbon, a carboxyl (-COOH or -COO-) group, an amine (-NH2 or -NH3+) group, and an "R" group Amino acids have the ability to be amphoteric •Amphoteric means that the amino acid has the ability to accept or donate protons (i.e., hydrogen ion) –Loss of a proton is called deprotonation –Addition of a proton is call protonation •The ability for an amino acid to either accept or donate hydrogen ions or protons is going to greatly impact the structure of the amino acid. In Summary: •In acidic pH (pH<7.0) –Carboxylic acid group is protonated (COOH) •No charge –Amine group is protonated (N+H3) •Positive charge •At neutral pH (pH=7.0) –Carboxylic acid group is deprotonated (COO-) •Negative charge –Amine group is protonated (N+H3) •Positive charge •At basic pH (pH>7.0) –Carboxylic acid group is deprotonated (COO-) •Negative charge –Amine group is deprotonated (NH2) •No charge Amino Acid Net Charge •Total net charge of an amino acid is extremely important to the functionality of the amino acid •The total net charge of the amino acid is calculated by adding together all the charges on the carboxyl group on the amine group, on the R group that are present in the amino acid. And it is this total charge that can greatly influence the amino acid’s functionality. Isoelectric Point One of the main reasons why we calculate the net charge of an amino acid is that it’s very important to the functionality of a food system. For example, let’s talk about the concept of the isoelectric point. The isoelectric point is the pH of the food environment in which the net charge of the amino acid is zero. When the net charge of the amino acid is zero, it’s actually at a minimal solubility so it actually has a really hard time staying within a solution. Sometimes this is actually very beneficial, for example in the production of cottage cheese. In making cottage cheese we actually acidify milk down to the pH at which the proteins within the milk will be at their isoelectric point and they will actually precipitate out making the curd in cottage cheese. However, sometimes it’s not very favorable and we as food scientists need to be able to realize the impact of the food environment on the overall net charge of the amino acid and how that can result in quality issues. A. Structure-function relationships –The R-group determines the functionality of the amino acid –The types of amino acids which comprise a protein determine the functionality of that protein •Example: Corn protein (zein) • •Example: Protein emulsifiers –Pairs of specific amino acids (dipeptides) taste very sweet and function as sweeteners in food systems •Example: Aspartame = L-phenylalanine + L-aspartic acid • •Example: Alitame = L-aspartic acid + D-alanine 6. Proteins: Structure and Functionality I. Protein structure A. Formation of amino acid polymers 1. Proteins are polymers of amino acids that are linked together by covalent bonds a. The bond links the carboxyl group (–COOH) of one amino acid to the amino group (–N H2) of another amino acid 2. The link is called a peptide bond and consequently, small proteins are called "polypeptides" 3. Peptide bonds are formed in condensation (water loss) reactions a. Types of linkages that create secondary and tertiary structures include: 1-4 4. Quaternary structure: the interaction of two or more proteins to form a new, larger protein structure a. Example: Hemoglobin II. Protein-Rich Foods A. Sources of protein-rich foods in the diet 1. Main plant sources of protein include: legumes, nuts, and cereals 2. Main animal sources of protein include: milk, cheese, eggs, meat, poultry, and fish 3. Protein-rich foods from animal sources have a higher biological value than those from plant sources III. Functionality of Proteins A. Functionality is determined by protein conformation 1. Proteins exist in one of two conformations: 2. Denaturation is the process of converting a protein from its naturally- occurring (or "native") conformation into a new conformation 3. Denaturation usually causes a structural transition from an ordered to a random conformation which affects the functionality of the protein in a food system 4. Denaturation affects all structures EXCEPT the primary structure of the protein 5. Denaturation is usually irreversible 6. Chemical or physical agents that denature proteins important in foods 7. Protein denaturation commonly occurs during food preparation and increases protein digestibility B. Specific functional properties of proteins in food systems 1. Moisture retention: 2. Gelation: 3. Emulsification: 4. Foaming: 5. Dough formation: a. Depends on the conversion of two protein fractions of wheat flour (gliadins and glutenins) into a functional protein complex called gluten b. Once developed gluten is stretchy yet elastic (called viscoelastic) 6. Texturization: a. Process: Extrusion (single-screw or twin-screw) b. Main ingredients: water, protein (provides structure), and starch (provides puffing) 6. Color formation: a. Amino groups (-N +H3) participate in Maillard (or non-enzymatic) browning with reducing sugars 7. Enzymes: 7. Enzymes: Biological Catalysts I. Definitions and mechanism of enzyme activity A. Definitions 1. Enzymes are proteins that Examples: 2. Enzymes are classified and named according to the type of biochemical reactions they catalyze Examples: 3. The molecule on which the enzyme acts is called a substrate B. Mechanism 1. Enzymes function to decrease the activation energy of a reaction 2. An enzyme-substrate complex is formed that is less stable than the substrate alone 3. The complex subsequently decomposes to the enzyme and products (QuickTime movie hyperlink) 4. The substrate molecule fits precisely into the active site of the enzyme (called a lock-and-key model 5. Enzymes are not permanently changed or destroyed during a reaction 6. The rate at which an enzyme catalyses a reaction is called the activity of the enzyme 7. Activity is highly dependent on: a. temperature b. pH optimum 8. Enzymes can catalyze both desirable or undesirable reactions in food systems II. Desirable enzymes: benefits and applications A. Beneficial enzyme attributes B. Enzymes have many applications in the food industry 1. Glucose isomerase: a. Example: 2. Invertase: 3. Proteases (papain, bromelain, and ficin): 4. Rennin (also called chymosin): 5. Pectinase: III. Undesirable enzymes A. Source of the undesirable enzymes 1. Nearly all raw food tissues (plants and animals) contain naturally occurring active enzymes 2. After harvest or slaughter, the enzymes continue to react on the available substrate B. Specific enzymes that may deteriorate the quality of raw and processed foods 1. Pectinases: 2. Pepsin: 3. Polyphenol oxidase (PPO): 4. Lipase: 5. Lipoxygenase: C. Combating enzymatic degradation of foods 1. Since all enzymes are proteins, undesirable enzymes in foods can be inactivated by applying chemical or physical agents that cause denaturation (1) Example: (2) Example: 2. Interfere with the enzyme-catalyzed reactions (1) (2) 8. Lipids: Structures, Properties, Types, and Uses I. Lipid classification and structure A. Free fatty acids 1. Classified by number of double bonds: a. saturated = FA containing the maximum number of hydrogen atoms b. unsaturated = FA containing less than the maximum number of hydrogen atoms, featuring at least one double or triple bond; can be monounsaturated or polyunsaturated chain: < 10 carbon atoms b. Medium chain: = 10–14 carbon atoms c. Long chain: > 14 carbons 3. Geometric shape: a. The R-groups bonded to the carbons in a double bond can exhibit one of two configurations: 2. Number of carbon atoms: a. Short (1) Cis = R-groups are positioned on the same side of the double bond; puts kink in molecule; common configuration in biological systems (2) Trans = R-groups are positioned on the opposite side of the double bond a. Delta (δ): the carbons are numbered from the carboxyl (- COOH) end of the FA Example: Linoleic b. Omega (ω): the carbons are numbered from the methyl (- CH3) end of the FA Example: Linoleic 5. Four FA comprise about 90% of the total FA that we consume in our diet B. Triglycerides 1. Contains two organic structures a. Glycerol "backbone": a water-soluble alcohol b. Fatty acids (FA): chains of carbon atoms with a carboxylic acid (–COOH) at one end and a methyl (–C H3) at the other end 2. The structures are linked by an ester bond (R–COOR') 4. About 95% of all lipids are triglycerides 5. Other glycerides are: a. Monoglyceride = glycerol + one fatty acid b. Diglyceride = glycerol + two fatty acids c. Phospholipids C. Phospholipids 1. Contains a glycerol + 2 fatty acids + (phosphate + a nitrogen base) D. Sterols 1. Large, intricate molecules consisting of interconnected rings of carbon atoms with a variety of side chains 2. The sterol of most significance in foods is cholesterol 3. Although both animal and plant foods contain sterols, cholesterol is only present in significant quantities in foods of animal origin II. Lipid properties A. Physical states 1. Lipids are classified based on physical state: a. Fats exist as solids or semi-solids at room temperature b. Oils exist as liquids at room temperature c. Explanation of physical states movie 2. Fatty acid composition determines physical state: B. Melting point 1. Melting point (MP) is a solid-to-liquid phase transition that usually occurs over a temperature range 2. The end point temperature is reported as the melting point of the lipid 3. Factors affect the melting point: a. Chain length: longer = higher MP b. Degree of saturation: more H atoms = higher MP c. Geometric isomerism: trans configuration = higher MP Example: Oleic acid (cis) MP = 16.3 °C and Elaidic (trans) MP = 45.0 °C III. Lipid types and uses A. Types of lipids used as food ingredients 1. Butter: 80% w/w milk fat, 16% water, and 4% milk solids 2. Margarine: 80% w/w hydrogenated vegetable oils (especially soybean and cottonseed oils) 3. Vegetable oil spreads: "Reduced-calorie margarines" that are 45 to 75% w/w hydrogenated vegetable oils 4. Hydrogenated fats: like Crisco; 65% of these products are made from soybean oil 5. Vegetable oils: extracted from soybeans, maize, canola, sunflower seeds, coconut, palm kernels, olives, peanuts, safflower seeds, cottonseeds, and sesame seeds 6. Cocoa butter: fat from the seeds of the Theobroma cacao tree; used in making chocolate; has unique melting properties 7. Animal fats: lard (from swine) and tallow (from beef or sheep) is separated from animal carcasses by rendering or heating in order to melt the fat and rupture fat cells so that the fat can be extracted 8. Fish oils: used for their omega-3 fatty acid content which may decrease this risk coronary heart disease and improve bone growth 9. No-calorie fat mimetics: Olean (Olestra) (Proctor & Gamble) (1) Development required 25 years of research and $200 million (2) Approved by the FDA for use as a fat mimetic in savory snacks in 1996 (3) Composed of a sucrose molecule with 6–8 fatty acids in place of – OH functional groups (ester linkages); consequently, the molecule is called a "sucrose polyester" (SPE) (4) The molecule is too large to be hydrolyzed by pancreatic lipase (5) It passes through the GI tract without being absorbed, so its caloric density is 0 kcal/g (6) SPE decreases the absorption of fat-soluble vitamins from the food into which it is formulated (7) When it was approved in 1996, the warning below was required on the label of products formulated with Olestra. As of August 1, 2003 the FDA concluded that the label statement is no longer warranted, however manufacturers are still required to add Vitamins A, D, E, and K. An asterisk after each of these lipid soluble vitamins references the statement, "Dietarily insignificant." "This product contains Olestra. Olestra may cause abdominal cramping and loose stools. Olestra inhibits the absorption of some vitamins and other nutrients. Vitamins A, D, E, and K have been added." (no longer required) B. Uses of lipids in food formulation and preparation 1. Lipids serve as carriers of fat-soluble vitamins, impart satiety to foods, and provide the flavor and texture of many foods 2. Fats and oils are used in frying processes 3. Spreads and salad dressings 4. Mono- and di-glycerides and phospholipids can be used as emulsifiers Section 3: Food Microbiology and Processing 1. Food Processing: Preservation, New Ingredients, and New Products I. Food processing A. Purposes 1. Food preservation a. The highest priority of the food industry is ensuring that our food supply is safe b. The key determinants of safety are pre- & post- harvest control of: (1) Food-borne pathogens Example: (2) Natural toxicants Example: (3) Added toxicants Example: (4) Food ingredients and additives Example: (5) Environmental contaminants Example: Food Defect Action Levels Handbook c. The secondary priority of the food industry is ensuring food quality d. The key determinants of food quality are: (1) Desirable aroma, flavor, and taste (2) High nutritional value Movie 21.1 (13 MB). Identify at least two reasons why canned produce may have a higher nutrient content than fresh produce. (3) Desirable appearance (size, shape, and color) (4) Desirable texture (5) Available variety (6) Convenience (7) Low price (8) Minimal environmental impact 2. Developing new ingredients and food products a. New ingredients b. New food products Movie 21.2 (13.8 MB). For a new product to be successful what must it do for the consumer? (1) Snack foods Example: (2) Shelf-stable products Example: (3) Novelty foods Examples: B. Methods of food processing 1. Chemical methods: addition or exposure of foods to chemicals for the purpose of extending shelf-life a. Example: 2. Biological methods: use of biological or microbiological organisms to create desirable functional molecular components a. Example: 3. Physical methods: a. Refrigeration and freezing b. Thermal processing (1) Canning (using various containers) (2) Aseptic processing c. Water management or removal 4. Non-thermal processing methods: a. Irradiation - Food irradiation is the process of exposing food to a specified amount of ionizing radiant energy using electrons, gamma rays or x-rays. This energy travels through the food killing pathogens, without raising the temperature of the food. The process also is referred to as "cold pasteurization" because bacteria are killed without the use of heat. Food irradiation is classified as a chemical method in the U.S. b. Pulsed electric fields - uses pulses of high voltage between two electrodes to create a high intensity electric field. The electric field causes pores in the membrane of the microorganism to develop, which inactivates the microorganisms by loss of cellular material. The advantage of processing foods using pulsed electric fields is that the process can be carried out at ambient temperature, resulting in safe, high quality foods. c. High pressure processing - Solid foods are packaged and placed in a vessel (liquids are pumped through) that is filled with water and pressurized in the range of 300 to 700 MegaPascals (500 MPa is equivalent to 20 family cars bearing down on an area the size of a postage stamp!). These pressures, maintained for a few minutes, can cause more than a 10,000 fold reduction in numbers of food poisoning bacteria. High pressure kills microorganisms by interrupting their cellular functions. The combined effect of high pressure with mild heating (less than 60 C) was found to be even more effective at increasing the microbial kill. High pressure processing results in safe, good tasting foods. 5. Packaging technologies: a. Modified Atmosphere Packaging (MAP) is the process that removes air (78% nitrogen, 21% oxygen, 0.03% carbon dioxide and traces of noble gases) from the package and replaces it by a desired gas (e.g., N2) or gas mixture (e.g., CO2 and N2) Examples: Fresh refrigerated pasta and Packaged cut lettuce b. Controlled Atmosphere Packaging (CAP) is the process that dynamically controls the atmosphere in a package either by external means or by the characteristics of the packaging materials used Example: Packaged fruits and vegetables c. Vacuum packaging is the removal of air from a packaged food Example: Retail cuts of fresh meat C. Examples of processed foods 1. Milk 2. Crystal Light powdered drink mix 3. Minimally processed fresh vegetables (e.g., pre-cut lettuce and baby carrots) 2. Microorganisms: Bacteria, Yeast, and Mold I. Microorganisms found in food A. General classification 1. The "good" microbes are those used to preserve foods and/or provide desirable properties to food materials 2. The "bad" microbes are those cause unacceptable defects in the appearance, flavor, texture of foods, but present no health hazards to consumers 3. The "ugly" microbes are those that cause food-borne illness (food infection or intoxication) II. Classes and characteristics of microorganisms A. Bacteria 1. Shape: rod (bacilli), spheres (cocci) Figure 22.1. The food pathogen Salmonella enteriditis is a rod- shaped bacteria. Figure 22.2. The food pathogen Staphylococcus aureus is a spherical bacteria. 2. Size: small, typically around 1 micron in length 3. Nuclei: does not contain a nucleus (prokaryotic organism) 4. Reproduction: asexual, capable of very rapid exponential growth 1) Lag phase 2) Log phase 3) Stationary Phase 4) Death Phase a. Example food safety calculation: If a food product is left out on the counter and contains one viable food infection type microorganism, how much time elapses before the food becomes unsafe for consumption? The average lag phase generation time is 60 minutes and the average log phase generation time is 20 minutes. Two generation times (4 microorganisms) are required for the system to switch from the lag to the log phase. For this particular microorganism, the number of microorganisms considered unsafe for consumption is 10,000. Lag: 60 Log: 20 There are 2 lag phases and 12 log phases 5. Spore formation a. Spore formers- Some are extremely heat stable b. 6. Temperature requirements: a. Psychrophiles: 0- 10 degrees C b. Mesophiles: 10-40 C c. Thermophiles: >40C d. Psychrotrophs: known as facultative psychrophiles have evolved to be able to grow at refrigerated temperatures 7. Oxygen requirements: a. Aerobes: require oxygen for growth b. Facultative anaerobes: can grow with or without oxygen c. Anaerobes: cannot grow in presence of oxygen 8. Pathology: a. Pathogenic: some cause food infections Example: salmonella b. Toxigenic: food intoxiactions Example: clostridium botulinum 9. By-products: a. Gases: hydrogen sulfide b. Small molecular weight compounds: acids, alcohols, aldehydes, ketones B. Yeast 1. Shape: a. b. 2. Size: larger than bacteria, typically 10 dm 3. Nuclei: contains a nucleus (eukaryotic organism) 4. Reproduction: can also produce sexually The saccharomyces cerevisiae can reproduce asexually by budding, forming a daughter cell from the mother cell. 5. Spore formation: fromers, non-spore a. 25-30C, VERY PICKY b. 6. Temperature requirements: 7. Oxygen requirements: a. Aerobic: best growth (bread) b. Anaerobic: slow growth (beer) 8. Pathology: none known 9. By-products: a. Gases: CO2 b. Small molecular weight compounds: alcohols C. Mold 1. Shape: many diverse shapes The mold spores grow into a mass of filaments called mycelia. 2. Size: large 3. Nuclei: contains a nucleus 4. Reproduction: asexual and sexual (budding) 5. Spore formation: heat stable 6. Temperature requirements: a. psychro- 0-10C b. meso- 25-30C c. thermo- >30C 7. Oxygen requirements: aerobic ONLY- grow on the surface of food products 8. Pathology: a. capable of producing bad toxins 9. By-products: a. Small molecular weight compounds: citric acid b. Large molecular weight compounds: antibiotics/vitamins III. What microorganisms need to grow and survive A. Important factors: FAT TOM 1. Food a. Nutrient availability b. Processing treatment(s) used c. Contamination 2. Acidity a. Bacteria (narrowest pH range) < yeast < mold (widest pH range) b. Most pathogenic organisms cannot develop in foods with pH < 4.6 3. Time 4. Temperature 5. Oxygen 6. Moisture Content (more specifically Aw) a. The classes of microorganisms differ in their need for water b. Bacteria (~ 0.90 Aw) > yeast (~ 0.86 Aw) > mold (~ 0.70 Aw) 3. Microorganisms: Food Fermentation I. Food fermentation A. Introductory concepts 1. The "good" microorganisms are used to preserve foods or provide other desirable sensory attributes to foods as a result of fermentation 2. Fermentation is one of the oldest processing and preservation techniques applied to foods a. Bread, beer, wine, cheese, fermented meats, and pickled vegetables were produced and consumed in the Biblical era 3. Fermentation involves the aerobic (with O2) and anaerobic (without O2) breakdown of carbohydrates (CHOs) a. Available complex CHOs or disaccharides are hydrolyzed by enzymes to glucose b. Glucose is then metabolized by the microorganism to produce many desirable organic products B. Classes of microbial food fermentations 1. Fermentation by bacteria a. Example: yogurt and cheese 2. Fermentation by yeast a. Example: bread, beer, and wine 3. Fermentation by both bacteria and yeast a. Example: sourdough bread 4. Fermentation by mold a. Example: blue cheese II. Food preservation through fermentation A. Basic principles 1. Microorganisms are allowed to reproduce in either a wild or controlled fermentation in the food 2. The microorganisms use available simple sugars, starch, or other nutrients for energy 3. Fermented foods are usually safe from further spoilage reactions or growth of pathogens. How? microorganisms give off byproducts that cause food to change and make it safer a. Fermentation produces acids, alcohol, and other organic molecules (such as natural antimicrobial compounds) and most spoilage organisms and pathogens cannot survive at low pH or in the presence of alcohol or antibiotics No growth of pathogenic microorganisms below pH of 4.6 b. In some fermented foods, air is excluded from the system so that undesirable aerobic organisms cannot develop during the fermentation process, such as molds III. Food processing through fermentation A. Classes of fermentations 1. Spontaneous or "wild" fermentations a. Microorganisms involved are those found in (or on) the raw food material b. Examples of foods produced by spontaneous fermentation: (1) Olives (2) Pickles (3) Sauerkraut (4) Tea (5) Coffee (6) Cocoa beans (7) Belgian lambic beers 2. Controlled fermentations a. The selected microorganisms are added into the food material as a large inoculum (starter culture) b. Examples of foods produced by controlled fermentation: (1) Wine (2) Vinegar (3) Beer (4) Buttermilk (5) Yogurt (6) Bread (7) Sausage (8) Cheese (9) Soy Sauce B. Fermentation process examples 1. Fermentations using bacteria: Swiss cheese Cheese making is a dehydration process, it influences the flavor and slows down interactions with microorganisms Components of whole milk Water Soluble Water, carbs (lactose), protein-whey, some vitamins, some minerals Lipid Soluble Lipids, Protein- casein, some vitamins Swiss cheese has a pale yellow color and a mild nutty flavor distinguished by large holes formed by CO2 gas produced during fermentation. 2. Fermentations using yeast: two purposes a. To produce alcohol (ethanol) in beer, wine, and for the starting material of fermented spirits (1) b. To produce leavening gas in baked products (1) Yeasts metabolize simple sugars and produce carbon dioxide gas and ethanol gas (2) The liberated carbon dioxide and ethanol gases, in addition to the water vapor, cause the bread dough to rise 3. Fermentations using mold, bacteria, and yeast: soy sauce Movie 23.1 (9 MB). What ingredients and processing steps are used to manufacture soy sauce? Wheat that is roasted, Soy is steamed, then you add the seed mold (allows for incubation time) which creates cogen, after about 3 days it goes into the fermentation tank where bacteria is added (yeast), fermented under controlled process- produces mash, goes into pressing to pull out mature soy sauce, then it is pasteurized. 4. Genetically Modified Foods: Methods, Regulatory Processes, and Potential Impacts Biotechnology Any technique that uses living organisms or substances from those organisms to make or modify a product, to improve plants or animals, or to develop microorganisms for specific uses. Genetic Instructions DNA (genetic material) works like computer software Changes to the program code Changes to function or appearance Genetic Variation Examples: Selective Breeding and Cross- Breeding Domestication led to adaption which led to genetic modification Modern Biotechnology Genetic engineering or modification Find a specific gene (set of instructions) that creates a desired trait Transfer the gene to the plant Where do these genes come from? Genetic Engineering Beneficial genes and traits from any organism may be used. GM targets ONE gene So… GMO = genetically modified organism GM = genetically modified GE = genetically engineered Uses of GMOs Pest Resistance: Non -GMO crop with corn borer damage vs. Bt corn resistant to Southwestern and European corn borer Sprays containing Bt bacteria have been safely used for 60 years as an organic pesticide Bt bacteria make a protein that is toxic to certain insects Why Has GM Been Adopted? Genetic improvement can be more efficient and precise compared to breeding Beneficial genes and traits from any organism may be tested for improvements over nature Effective delivery of agricultural solutions via seeds rather than external inputs Fewer pesticide applications… $ savings on labor, chemical, and energy costs Decreased chemical exposure for laborers Better nutrition Development and Approval Substantial Equivalence • Demonstrate that GM variety not significantly different • Differences can exist – are they biologically meaningful? Process can take 7-10 years! Nutrient content • Must be the same or better (e.g., Golden Rice) Toxicity and potential to trigger allergies • Proteins • Gene source Substantial Equivalence Nutrient content • Same or better Potential to be toxic • Proteins • DNA ingestion • Gene source Potential to trigger allergies • Proteins • Gene source DNA/Protein Digestion DNA = similar to protein in its breakdown DNA is broken down to its components similar to how protein is digested to amino acids; ingesting DNA has never been found to be toxic Types of Studies Methods are similar to those used when testing crop chemicals and new drugs • Compositional studies – compare to a known product • Digestibility – how fast is protein broken down? • Oral toxicity tests – purified protein in mice/rat models • Animal feeding studies – livestock, poultry, fish Monitoring Safe to grow? • Crop invasiveness • Effects on other crops/organisms • Changes over time Safe for environment? • Evaluates pesticidal substances made by crops Pesticide and Herbicide Resistance Superpests and Superweeds? Some evidence for this – why is it happening? • Resistance is a concern for ALL crops Over the past 20 years, pesticide applications worldwide have decreased by 37% Studies were included when they build on primary data from farm surveys or field trials anywhere in the world, and when they report impacts of GM soybean, maize, or cotton on crop yields, pesticide use, and/or farmer profits. In total, 147 original studies were included. Overall herbicide usage has not increased Increased glyphosate use: • Wider adoption of Roundup Ready crops • Emerging resistance? Environmental Changes Doses of chemicals used in lab experiments Likely exposure in natural setting (fields) No documented impact of GM crops on butterflies and bees Environmental effects can happen when any new crop is introduced USDA/EPA monitoring Diseases and Disorders Have GMOs led to higher rates of allergies, cancer, autism, and other health issues? GM foods are not causatively linked to allergies, cancer, infertility, ADHD, or other diseases. Correlation vs. Causation Global Response to GMOs Each country has its own certification process Very few countries explicitly ban GMOs • May not yet have regulations in place • Cultivation vs importing • Foreign policy • Some have mandatory food labeling Labeling U.S. system is based on defined food safety risk No evidence to suggest safety concerns No (mandatory) need to label Right to Know? Concerns over mandatory labeling • Lack of consumer understanding • Implies food is different and potentially unsafe • Could increase food costs (testing, product replacement) 5. Microorganisms: Food-Borne Illnesses I. Categories and causative organisms of food-borne illnesses A. Categories of food-borne illnesses 1. Food infection a. Consuming a food contaminated with viable microorganisms that can multiply in our gastrointestinal tract and produce the symptoms of illness b. The number of microorganisms required to cause a food infection varies from very few (e.g., 10 for E. coli O157:H7) to very many (e.g., thousands for Campylobacter jejuni) c. Can be caused by bacteria, viruses, parasites , and protozoans d. Susceptible populations: d. Multiple shiga-toxin producing E. coli have are zero tolerance microorganisms: O157:H7, O26, O45, O103, O111, O121, and O145 (2) Most E. coli are harmless inhabitants of the intestinal tract, but once in the human gut, some variants produce toxins that are capable of deadly damage. These toxins damage the cells of the intestinal lining and results in bloody stools. In some cases (2 to 7% usually the young and the elderly), the toxin enters the patient's bloodstream through the damaged intestinal wall and causes kidney failure, specifically hemolytic uremic syndrome 2. Food intoxication a. Consuming a food contaminated with toxins produced by microorganisms during reproduction and development b. The microorganisms need not be viable in order for illness to occur c. Food-borne toxins can be produced by bacteria or mold d. Claviceps purpurea and the Salem Witch Trials II. Incidence, costs, and prevention of food-borne illnesses A. Where do food-borne illnesses occur? 1. In homes and restaurants. Approximately what percentage of kitchen sponges may contain pathogenic microorganisms? According to this news story what is the best way to sanitize a kitchen sponge? 2. In food processing plants B. How common are food-borne illnesses? 1. Frequency a. 76 - 81 million people become sick every year 2. Mortality a. 3. Validity of statistics a. The incidence of food-borne illness is probably under-reported since the current CDC surveillance system in the U.S. reports only a fraction of the cases of food-borne illness that actually occur. b. The common affliction known as the "24 hour flu" is often due to food-borne illnesses 4. Complications a. The Food and Drug Administration (FDA) estimates that about 2 to 3 percent of all foodborne illness cases lead to serious secondary long- term illnesses. C. How costly are food-borne illnesses? 1. Major economic losses (in food processing and food service sectors) are associated with food-borne illnesses a. Loss of customers, sales, and business reputation b. Lower employee morale c. Need to retrain employees and develop and implement new processing quality standards d. Product recall and associated legal costs e. Increased insurance premiums f. Health care costs 2. USDA Economic Research Service (ERS) estimates the yearly cost of salmonellosis is $2.65 billion (2010). In 1993, they estimated the total cost of foodborne illness to be between 5.6 and 9.4 billion dollars. D. Fighting food-borne illness 1. The Fight BAC Campaign: Four simple steps to safe food Clean, separate, chill, and cook 6. Food Preservation: Refrigeration and Freezing I. Definitions A. Refrigeration- Provides cool storage of foods at temperatures ranging from 28 to 60 F or -2 to 16 C. To be out of the Danger Zone, food temperature need to be less than 41 F. 1. Water in the food is not frozen Consequently, the shelf life of perishable products is extended only for a few days or weeks 2. Growth of nearly all pathogenic ("ugly") microorganisms is prevented a. However, some spoilage ("bad") microorganisms (psychrophiles) may thrive B. Freezing 1. Provides cold storage of foods as temperatures lower than 28 F. Typically at temperatures 0 F or -18 C or colder. 2. Most of the water in the food (about 95 to 98%) is frozen a. Consequently, the shelf life of perishable products is extended for months to years b. The rate of freezing is affected by several factors that may be controlled by the food manufacturer 3. Growth of all microorganisms is prevented and some are destroyed Growth of all microorganisms is prevented and some are detroyed 2 Movie 27.1 (3 MB). What factors influence the rate at which foods can be frozen to zero degrees Fahrenheit? Temperature of the food, density, moisture content, packaging material, size of the carton, Salt and sugar content, air temperature and velocity Movie 27.2 (7 MB). How do packaging materials affect the rate at which foods freeze? The thinner the package material, the heat transfer will happen more easily and will be more efficient. Products that are directly exposed to the air will be more immediate for freezing. Small particles in a big box, air acts as an insulator so it won't freeze as fast. II. How refrigerators and freezers operate A. Refrigerators 1. Function as heat pumps removing (or transferring) heat from the food 2. Contain four essential mechanical components a. Evaporator- Heat from the food changes liquid refrigerant into gaseous refrigerant b. Compressor- The temperature and pressure of the gaseous refrigerant is increased c. Condenser- Gaseous refrigerant condenses back into a liquid when the heat in the refrigerant is released from the back of the refrigerator d. Expansion valve- Meters refrigerant into the evaporator When Foods Are Frozen What Happens to the water in the plant and animal tissues? 11 percent increase in volume. Causes tons of cellular and tissue damage. B. Freezers 1. Use the same four components as above, but the refrigerant can be applied to the food indirectly (e.g., a plate freezer) or directly (e.g., liquid nitrogen) in order to change most of the liquid water in the food material to solid ice. When ice crystals ripen at constant temperature what changes can be observed in their relative size and number? III. Effects of refrigeration and freezing on foods A. Refrigeration 1. Desirable effects a. Microbial growth rates decrease (1) Example: milk, leftovers b. Chemical reaction rates decrease (1) Example: Vitamin C loss, lipid oxidation c. Biochemical reaction rates decrease (1) Example: enzyme reaction rates, respiration d. Shelf life increases (2 to 5 fold for every 10 °C decrease in temperature) 2. Undesirable effects a. Textural deterioration (1) Example: staling of bread b. Chill injury (1) Example: bananas B. Freezing 1. Desirable effects a. Microbial growth eliminated (0 °F [-18 °C]) (1) Example: raw meats, poultry, and fish b. Chemical reaction rates decrease significantly (0 °F [-18 °C]) (1) Example: lipid oxidation c. Biochemical reaction rates decrease significantly (0 °F [-18 °C]) (1) Example: enzyme reaction rates d. Shelf life significantly increases (3 to 40 fold for every 10 °C decrease in temperature) 2. Undesirable effects (chemical or physical processes, not microbial) a. Chemical deterioration (1) Example: pigment washout b. Physical (textural) deterioration (1) Example: crystal formation in ice cream IV. Energy removal resulting from refrigeration and freezing A. Temperature versus time diagram B. Energy removal calculations 1. Refrigeration a. Removal of heat (Q) from Region I (sensible heat) (1) Q = Weight of food x specific heat of food above freezing x temperature difference b. Cooling of 1 pound of peas (85% mc) with a specific heat above freezing of 0.75 BTU/lb °F from 75 to 40 °F (1) Q = 2. Freezing a. Removal of heat (Q) from Region I (sensible heat), II (latent heat), and III (sensible heat) (1) Q = Weight of food x specific heat of food above freezing x temperature difference (2) Q = Weight of water x latent heat (3) Q = Weight of food x specific heat of food below freezing x temperature difference b. Freezing of 1 pound of peas (85% moisture content) with a specific heat above freezing of 0.75 BTU/lb °F and 0.40 BTU/lb °F below freezing from 75 to -5 °F. The freezing point of the peas is 25°F. The latent heat of water is 144 BTU/lb. (1) Q = (2) Q = (3) Q = Example Questions How much energy must be removed to refrigerate 2 pounds of ground turkey with a specific heat above freezing of .54 BTU/lb F from 50 F to 38 F? Q1= 2 lb x .54 BTU/lb F x (50-38 F) = 12.96 BTU Q Total- Add Q1 Q2 and Q3 How much energy must be removed when freezing 2 pounds of ground turkey (46 percent moisture content) with a specific heat above freezing at .54 BTU/lb and a specific heat below freezing of .36 from 50 F to -8 F? The freezing point of the turkey is 27 F. The latent heat of the water is 144 BTU. Assume all the water freezes. 50- 27 F= 24.84 BTU 2 lb x .46 x 144 BTU/lb= 132.48 BTU 2 lb x .36 BTU/lb x (27- -8)= 25.2 BTU we have already taken the food down to 27, so don't start at 50 Add them all together= 182.52 7. Food Preservation: Thermal Processing I. Thermal processing of foods A. Purposes 1. Purpose to inactivate enzymes and metabolic reactions resulting in senescence Normal, non-microbial degradation of plant tissue prior to tissue death 2. To destroy pathogenic microorganisms and their spores B. Trade-offs 1. High temperatures can diminish product appearance, texture, and nutrient quality 2. Food scientists must optimize the advantages of thermal processing (food safety and increased shelf life) with the disadvantages (decrease in some product nutrient and sensory attributes) II. Methods of thermal processing A. Variation in treatment temperature and time 1. Blanching a. Mild heat treatment, usually applied to fruits and vegetables to denature enzymes prior to freezing Example: green beans b. 2. Pasteurization a. Process developed by Louis Pasteur in approximately 1864 b. Destroys pathogenic microorganisms and extends the shelf life of food c. Pasteurized products still contain many viable organisms capable of growing and causing spoilage defects (1) Example: Milk d. Usually pasteurization is combined with another means of preservation like refrigeration e. Levels of pasteurization used to thermally process milk: (1) Low Temperature Long Time (LTLT): 145 F for 30 min (2) High Temperature Short Time (HTST): 161 F for 15 sec (3) Ultra High Temperature (UHT): 280 F (or higher) for 2 sec***LTLT and HTST are equivalent thermal processes 3. Commercial sterilization a. Process developed by Nicolas Appert and published in 1810 b. All pathogenic and toxin-forming organisms are destroyed, as well as some spoilage microorganisms c. These foods may contain a small number of heat resistant bacterial spores d. Types of commercially sterile processes: (1) Processing diagram e. Most commercially sterile food products have a shelf life of 2 years or longer 4. Sterilization a. Complete destruction of all microorganisms, including both vegetative cells and spores III. Heat resistance of microorganisms A. Effects of thermal processing 1. Bacteria are killed by heat at a rate that is nearly proportional to the number present in the food being heated a. Called the logarithmic order of death b. Expressed as the "D-value," or decimal reduction time 2. D-value: The time in minutes at a specific temperature required to destroy 90% (or one log cycle) of the organisms in a population a. Example: D-value for Campylobacter jejuni in ground chicken = 2.25 mins at 131 °F 3. The amount of time (or number of D-values) required depends on the: a. pH b. temperature c. viscosity 4. Examples of the thermal death rate: A. If we start out with 1,000,000 viable, pathogenic microorganisms and the D-value for killing this pathogen is one (1) minute at 250 °F, how many minutes would it take to reduce the population to 1 viable cell? Short Hand. 1 0 0 0 0 0 0 Calculate how many times you have to move the decimal place to get to what you want. Move it 6 times. 6 decimal reduction x 1 min/decimal reduction. tes B. You start out with 10,000 viable, pathogenic microorganisms and after 15 minutes at 230 °F you have reduced the microbial load to 0.1. What is the Dvalue for this process? 1 0 0 0 0 How to get to 0.1? Move it 5 times. 15 minutes/ 5 decimal reductions= 3 minu 5. A "12D process" (when 1 in 1 trillion cells survive) is commonly used in commercial canning 6. Equivalent processes – different temperature-time combinations that are equally effective in microbial destruction Example: C. botulinum destruction in low-acid media 0.78 min at 127 C 10 min at 116 C 330 min at 100 C IV. The heat transfer process A. What is adequate heat processing? 1. Assuring that EVERY particle of food reaches the specified temperature for the required amount of time 2. The cold point in a can or food mass is the location that is last to reach the final heating temperature a. Use a thermocouple to determine location of the cold point B. Important factors for assuring adequate processing 1. Size, shape, and container material 2. Ingredients and pH of the food product 3. The state (solid or liquid) of the food product Determines the mechanism of heat transfer C. Mechanisms of heat transfer 1. Radiation: infrared energy is absorbed by food (surface heating), resulting in a temperature increase 2. Conduction: direct contact with heat source; heat move from one particle to another by direct contact 3. Convection: involves movement in the mass being heated; the heated portion of the material (occurs in liquids and gases, but not solids) become less dense and rises, setting up convection currents within the can and accelerates heat transfer D. Temperature-time combinations 1. Different temperature-time combinations can be equally effective in microbial destruction, but can differ in their effects on the nutrient and sensory attributes of foods 2. Higher temperatures facilitate use of shorter processing times for microbial destruction, and shorter processing times favor retention of desirable quality attributes 8. Food Preservation: Water Removal I. Removing water from food systems A. Categories of water removal 1. Recall that all foods contain at least some water 2. Partial water removal is called: 3. Nearly complete water removal is called: B. Purposes of water removal 1. To decrease the water content and water activity (Aw) of a food material a. Increases the microbial stability and chemical stability of the product (1) Example: 2. To decrease the shipping weight and/or volume of a food material a. Decreases the distribution costs and retail costs of some foods (1) Example: 3. To decrease the freezing costs of a food material a. Decreasing the water content requires removal of less energy and enables the food to freeze more rapidly (1) Example: II. Heat and mass transfer A. General mechanism 1. of the food material 2. Important variables to consider when using heat to remove water from foods: a. b. c. d. e. 3. A typical drying curve (moisture content versus time) a. The drying curve has two distinct parts: (1) Nearly linear relationship between moisture content and drying time (Points 1–2) (2) Non-linear relationship between moisture content and drying time (Points 2–3) 4. A typical drying rate curve (drying rate versus time) a. The drying rate curve has two distinct parts: (1) Constant rate period of drying (Points 1–2) (2) Falling rate period of drying (Points 2–3) III. Concentrated food systems A. Product characteristics 1. The amount of water removed, as well as the product moisture content, can vary greatly: 2. Jams, jellies, and syrups are examples of concentrated foods that resist microbial spoilage a. Why does this happen? -Water activities (Aw) of these products are reduced below ranges that support microbial growth -Due to high concentration of simple sugars 3. Concentration is usually the first step applied to liquid foods that will eventually be dehydrated a. In the water removal process, liquids are concentrated more economically by high-efficiency evaporators than in dehydrators (1) Example: 4. Foods may be modified in several ways (positive [+] and negative [-]) during the concentration process a. Decrease in water content and Aw b. Develop darker colors and cooked flavors c. Sugar crystallization d. Protein denaturation B. Processing methods 1. Solar concentration Movie 29.1 (14 MB). What specific chemical changes occur in grapes as they are sun dried in vineyards to produce raisins? 2. Open kettles a. Used for maple syrup where high heat is desired for producing color from caramelized sugar and developing characteristic flavors 3. Evaporators a. Moisture content is decreased by evaporating some of the water from the liquid usually using a steam jacketed single or multiple stage evaporator 4. Freeze-concentration a. Removal of water as ice crystals from the food system 5. Ultrafiltration and reverse osmosis a. Removal of water using membrane technology IV. Dehydrated food systems A. Product characteristics 1. Approximate moisture content and Aw of dehydrated products: a. Examples: (1) Dry (or powdered) milk (2) Cereals (3) Snack foods (4) Dry soups (5) Spices (6) Drink mixes (7) Instant coffee and tea (8) Dried pet foods (9) Pasta (10) Most "add-water-and-prepare" type foods 2. Microbial and chemical stability 3. Rehydration properties a. Appearance, flavor, and texture of the rehydrated food should be similar to that of the fresh material b. Hardening of the outer layer of the food is a common textural defect (called case hardening) 4. Product integrity a. Depends on how the dehydrated product will be used 5. Use of moisture-proof packaging to prevent adsorption of water from the atmosphere during distribution and storage 6. Question: Why are food products that are 100% solids and have an Aw > = 0.0 undesirable? B. Processing methods 1. Traditional drying a. Water removal is accomplished by placing a food in the sun, over a fire, or in smoke (1) Example: 2. Air convection dryers a. A stream of hot air in a closed environment supplies the heat by convection and carries away the evaporated water 3. Drum or roller dryers a. Liquid or paste foods applied on surface of hot stainless steel rolls; the dry material is scraped off the rotating rolls at the end of a revolution with a knife 4. Spray dryers a. Fluid foods are sprayed into a stream of hot air called (atomization); dry material falls to the bottom of the chamber and is removed 5. Freeze dryers a. Water is removed by sublimation from frozen foods under high vacuum; heat is supplied by conduction or radiation through the dry food layer b. Produces the best quality product, but is the most expensive Section 4: Food Laws, Quality, and the Consumer 1. Food Laws & Regulations I. Definitions and purposes A. Laws 1. The legislative branch of the federal government (Congress) establishes general policies and guidelines which govern the activities of the food industry 2. Legislation passed by Congress becomes law when the President signs it 3. Only Congress can amend the laws B. Regulations 1. The executive branch of the federal government is responsible for enforcing the laws through regulations 2. Regulations are created, modified, or revoked by the rule making process 3. Regulations may also be influenced by petition C. Purposes of food laws and regulations 1. 2. 3. II. The rule making process and petitions A. Steps involved (published in the Federal Register) in the rule making process 1. Advanced Notice of Proposed Rulemaking a. b. 2. The Proposed Rule a. 3. Comment Period b. Comments on the proposal to be filed for consideration before drafting the Final Rule are requested (minimum of 30 days) a. The agency presenting the Proposed Rule is required by law to consider and respond to each comment filed 4. Final Rules and Regulations a. Includes the agency's response to the comments, complete text of the Rule, and an effective date for enforcement b. The terms "rules" and "regulations" have the same meaning within the Federal Register publication system. 5. Force of the Law a. Regulations or Rules that have been promulgated as described above have the full force of the laws passed by Congress b. Guidelines are suggestions and do not have the full force of the law B. Petitions 1. Agencies can be petitioned to issue, change or cancel a regulation, or to take other action 2. Petitions must contain: 3. Most petitions come from industry or consumer groups, only a few come from individuals 4. The FDA receives hundreds of petitions yearly III. Enforcement A. Government agencies involved 1. Food and Drug Administration (FDA): primary responsibility is to ensure the safety and proper labeling of most foods through administrative enforcement powers (i.e., warning letters, adverse publicity, recalls, and withdrawals of product approval) 2. United States Department of Agriculture (USDA): responsible for ensuring the safety and labeling of meat and poultry products 3. Alcohol and Tobacco Tax and Trade Bureau (TTB) in the U.S. Department of the Treasury: regulates alcoholic beverages 4. Environmental Protection Agency (EPA): responsible for ensuring that pesticides applied to crop foods are safe 5. National Oceanic and Atmospheric Administration (NOAA): responsible for seafood quality and identification, fisheries management and development, habitat conservation, and aquaculture production 6. Centers for Disease Control and Prevention (CDC): responsible for responding to food-borne illness emergencies 7. U.S. Department of Justice (USDJ): responsible for judicial enforcement actions on behalf of the FDA (i.e., product seizure when foods are processed or packaged in violation of federal law; injunctions; prosecutions) 8. Federal Trade Commission (FTC): regulates food advertising III. History of federal food laws and regulations A. 1906 Pure Food and Drug Act (PFDA) 1. Prohibited interstate commerce of: a. Adulterated foods (1) Economic adulteration - adding a less valuable material (e.g., water to wine) or removing a more valuable material (e.g., fat from milk) (2) Foreign material - adding an aesthetically displeasing or potentially harmful material b. Misbranded foods (1) False or misleading statements (e.g., name, content, weight) 2. Prompted in part by Upton Sinclair's exposé, The Jungle (published in 1906) which described in explicit detail the adulteration that was common in the meat packing industry in Chicago Excerpt from The Jungle: "These rats were nuisances, and the packers would put poisoned bread out for them; they would die, and then rats, bread, and meat, would go into the hoppers together. This is no fairy story and no joke; the meat would be shoveled into carts, and the man who did the shoveling would not trouble to lift out a rat even if he saw one - there were things that went into the sausage in comparison with which a poisoned rat was a tidbit..." B. 1938 Federal Food, Drug and Cosmetic Act (FFDCA) 1. Superseded the 1906 Act 2. Underlying principle: the American consumer is entitled to a safe food supply and the opportunity to make informed decisions based on accurate information in the marketplace 3. The 1938 FFDCA is the foundation for contemporary food law because it: a. Broadened prohibition against economic adulteration b. Authorized the establishment of definitions and quality standards for food (1) Example: Imitation foods must be labeled as such c. Prohibited the use of misleading containers d. Required 4 items for affirmative labeling (1) Name of food (2) Net quantity of contents (3) Ingredient statement (4) Name and address of manufacturer, packer, or distributor e. Required additional information on dietary foods f. Prohibited false or misleading statements on labels g. Authorized plant inspections 4. The 1938 FFDCA has been "fine tuned" by amendments and supplemented by new laws, but after more than 60 years it remains the basis of a safe food supply C. 1958 Food Additives Amendment (amendment to 1938 FFDCA) 1. Established the Generally Recognized As Safe (GRAS) list (FDA's responsibility) and new food additives approval process (safety testing is the manufacturer's responsibility) 3. Contains the Delaney Clause a. "No additive shall be deemed to be safe if it is found to induce cancer when ingested by man or laboratory animals or if it is found, after tests which are appropriate for the evaluation of the safety of food additives, to induce cancer in man or animals." b. Problems with clause: a zero cancer risk standard with no provision for levels or amounts D. 1990 Nutrition Labeling and Education Act (NLEA) (amendment to 1938 FFDCA) 1. History of the Act a. Two main factors played a role in the history of the 1990 NLEA: (1) Changes in FDA philosophy (2) FDA was losing control of the food label b. FDA wanted to use the 1938 FFDCA to address the problems, whereas Congress wanted to add an amendment to the 1938 FFDCA c. Congress "won" 2. Requires all packaged foods to bear Nutrition Facts labels 3. Defined serving size and food label terminology (nutrient content claims) 4. Set criteria for approval (by FDA) and use of health claims on labels E. 1994 Dietary Supplement Health and Education Act (DSHEA) 1. Act established dietary supplements as a legal food category (called dietary ingredients) and a new set of requirements for their regulation 2. Dietary ingredients are not subject to either the food additive or drug approval process 3. Manufacturers wanting to market a new dietary supplements ingredient, not marketed prior to Oct 15, 1994, must submit a notification to the FDA at least 75 days prior to the expected marketing date 4. Nutrient content claims (must adhere to FDA definitions), health claims (requires FDA approval), and structure function claims (does not require FDA approval) can be made for dietary supplements (but not traditional foods) 5. The manufacturer is responsible for ensuring the accuracy and truthfulness of these structure-function claims a. If a structure-function claim is made the label must contain the following "disclaimer" statement: "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease." b. Manufacturers making a structure-function claim must submit a notification to the FDA no later than 30 days after marketing the dietary supplement 6. Differences between 1990 NLEA for traditional foods and 1994 DSHEA for dietary ingredients: a. Allowed claims on label b. Approval process F. 1996 Food Quality Protection Act (FQPA) 1. Establishes a single, health-based standard that eliminates previous regulation problems posed by multiple standards for pesticides in raw and processed foods 2. Uses "a reasonable certainty of no harm" as the general safety standard a. Replaced the Delaney Clause 3. Includes special protections for infants and children when assessing pesticide safety G. 2002 Public Health Security and Bioterrorism Preparedness and Response Act (Bioterrorism Act) 1. Purpose: to improve the ability of the U.S. to prevent, prepare for, and respond to bioterrorism and other public health emergencies 2. The Bioterrorism Act is divided into five titles a. The FDA is responsible for carrying out Title III -- Protecting the Safety and Security of the Food (Subtitle A) and Drug (Subtitle B) Supply 3. Main focus of Subtitle A - protection against intentional adulteration of food 4. Unless exempted, the provisions of Title III. Subtitle A apply to all types of food products regulated by FDA, including dietary supplements and animal feed H. Food Allergy Labeling and Consumer Protection Act 1. Purpose: to CLEARLY label foods that contain harmful allergens 2. Eight major food/food group categories for allergens (account for ~ 90% of all food allergies) a. Milk b. Eggs c. Fish d. Crustacean shellfish e. Tree nuts f. peanuts g. wheat h. Soybeans I. Food Safety Modernization Act 1. Purpose: to shift food safety from a reactive program to a proactive program 2. 3. 2. Sensory Science: Evoking and Measuring Sensations from Food I. Sensory science and the food industry A. Food Marketing Institute survey 1. The most important purchase criteria used by head of household grocery shoppers is product taste 2. Other criteria also influence food product purchase decisions a. Examples: 3. Assuming that these criteria are fulfilled, if the taste is not appealing, then the consumer will not purchase the product again B. Taste preference is not universal 1. Dietary preferences differ among groups of consumers based on a. Ethnicity, culture, and religion b. Age c. Geographic location 2. Although food scientists commonly use instruments to measure specific attributes of food products, there is no analytical means to measure consumer preferences for foods 3. Consequently, consumers (human and animal subjects) are used in sensory testing II. The science of the senses A. Definition of sensory science 1. "A scientific discipline used to evoke, to measure, to analyze, and to interpret reactions to those characteristics of foods and materials as they are perceived by the..." senses (IFT 1981) B. Use of sensory science 1. Sensory science uses formal experimental design and statistical methods to evaluate the perception of foods and materials by the senses III. The human sensory system A. Primary senses 1. Sight (Visual) 2. Touch (Tactile) 3. Smell (Olfactory) a. Nose 4. Taste (Gustatory) a. Tongue and Taste buds 5. Hearing (Auditory) 6. Common Chemical Sense (Chemesthesis) a. Compounds that stimulate trigeminal nerve endings in the mucosa of the eyes, nose, and mouth b. Examples: capsaicin in red chili pepper, piperine in black pepper, zingerone in ginger, isothiocyanate in mustard and horse radish, and 1- propenyl sulfenic acid in onion and garlic B. Fundamental tastes 1. Five fundamental tastes sensed by the taste receptor cells on the tongue a. Bitter b. Sour c. Salty d. Sweet e. Umami 2. Based on research reported in the late 1800's, it was thought that fundamental tastes are perceived on different regions of the tongue. Based on this concept a "Taste Map" was created (Figure 37.1) and continues to appear in the literature. However, further research has shown that all qualities of taste can be elicited from all regions of the tongue that contain taste buds. 3. "Flavor" is an attribute of foods that is a composite of the smell and taste sensations 4. The tongue of healthy adults has an estimated 10,000 taste buds, which are the receptors for tastes 5. Taste buds "wear out" and are replaced about every 7 to 10 days 6. The number of taste buds and the frequency of replacement decreases with age IV. Sensory testing A. Categories of tests 1. Analytical sensory tests a. Used by food manufacturers for internal evaluation of differences or similarities among sample products (1) Identification: what appearance/flavor/texture attributes are present? (2) Quantification: how much of each attribute is present? b. Experienced ("trained") panelists evaluate the samples c. The relative number of panelists is small (5 to 15) d. Example: Descriptive analysis test 2. Affective sensory tests a. Used by food manufacturers for external evaluation consumer acceptance or preference among sample products (1) Preference: which sample is preferred? (2) Acceptance: how much is a sample liked? b. Inexperienced ("consumer") panelists (screened and selected for desired demographics) evaluate the samples c. The relative number of panelists is large (usually > 50) e. Levels of consumer sensory tests (1) Focus groups (2) Central location tests (CLT) (3) Home use tests (4) Test marketing f. Buyers versus Consumers: How are purchase criteria influenced? (1) Examples: Adults and their children, owners and their pets 3. Good Manufacturing Practices (GMP) and HACCP I. Good Manufacturing Practices (GMP) A. Legal basis 1. The Federal Food, Drug, and Cosmetic Act (FFDCA) of 1938 gives the FDA the authority and responsibility for ensuring that the food supply in the U. S. is safe and wholesome 2. The goal of GMP regulations is prevention of food adulteration B. Regulatory history 1. Regulations are based on the 1938 FFDCA 2. Regulations were finalized during the late 1960s 3. Regulations contain "umbrella" GMPs (Title 21 CFR Part 110) a. Categories covered under the "umbrella" GMP (Title 21 CFR Part 110) 1) General provisions (including Personnel) 2) Buildings and Facilities 3) Equipment 4) Production and process controls 5) Defect action levels b. Three specific industry GMPs for: 1) Thermally processed low-acid canned foods 2) Acidified foods 3) Bottled drinking water ***FDA has also published a voluntary food safety guideline (similar to a specific GMP) for minimizing microbial food safety hazards in fresh fruits and vegetables II. Hazard Analysis and Critical Control Points (HACCP) A. Basis 1. HACCP is a science-based system with a goal to ensure that food safety hazards are controlled to prevent unsafe food from reaching the consumer B. Regulatory history 1. HACCP was developed for foods used in the space program through a cooperative effort among four groups: a. The Pillsbury Company b. National Air and Space Administration (NASA) c. U.S. Army Natick Research Center d. U.S. Air Force Space Laboratory Project Group C. Seven elements of an effective HACCP program 1. Identify potential hazards a. Potential hazards associated with a food and measures to control those hazards are identified. The hazard could be biological, such as microorganisms; chemical, such as a pesticide; or physical, such as glass or metal fragments. 2. Identify critical control points (CCP) a. These are points in a food's production - from its raw state through processing and shipping to consumption by the consumer - at which the potential hazard can be controlled or eliminated. Examples are cooking, cooling, packaging, and metal detection. 3. Establish limits for critical control points a. For a cooked food, for example, this might include setting the minimum cooking temperature and time required to ensure the elimination of a particular microorganism. 4. Monitoring critical control points a. Such procedures might include determining how and by whom cooking time and temperature should be monitored. 5. Proper and effective corrective action a. For example, reprocessing or disposing of food if the minimum cooking temperature has not been met. 6. Record keeping system a. This would include records of hazards and their control methods, the monitoring of safety requirements and action taken to correct potential problems. 7. Verification system a. For example, testing time-and-temperature recording devices to verify that a cooking unit is working properly. D. HACCP video E. Current status 1. HACCP has become a regulation in the: a. Seafood industry b. Meat and poultry industry c. Juice industry 2. The Codex Alimentarius Commission (an internationa]l food standard- setting organization) has adopted HACCP as the international standard for food safety (included in a World Health Organization publication, 1993). III. GMP and HACCP programs A. Summary points 1. By complying with GMP and adopting a HACCP system, food manufacturers share the responsibility of ensuring a safe food supply with FDA 2. Prevention of hazards through the use of GMP and HACCP programs is better than detection of hazards in the finished product 4. Quality Assurance in the Food Industry I. Management of quality A. Historical chronology 1. 1850 Pre-Industrial Revolution: Quality "controlled" by the individual craftsman who was involved in all aspects of the products life cycle 2. 1875 Frederick W. Taylor and "Taylorism" a. Concepts and methods of division of labor and mass production begin to appear in the American industrial sector b. Two problems evolved: (1) A decrease in product quality (workmanship) (2) Only a moderate increase in productivity c. Two plans (solutions) were developed: (1) Work standards (2) Wage incentives d. Did these plans address both problems? 3. 1924 Walter Shewhart of Bell Labs introduces statistical process control (SPC) in the form of control charts a. Methods based on the continual on-line monitoring of process variation b. Focus is on process control for end-product quality 4. 1930s Dodge and Romig of Bell Labs introduce double (acceptance) sampling methods a. Developed a lot-by-lot sampling inspection method for determining product suitability for shipment to the customer b. Methods are based on a probabilistic approach to the prediction of the lot character based on sampling results c. Focus is on product control for end-product quality 5. 1950s Dr. W. Edwards Deming's approach: quality AND productivity improvement a. A statistically-based approach to quality AND productivity improvement patterned after the work of Shewhart b. Critical to this approach is the involvement of top management c. Deming is ignored by U.S. corporate management but is accepted readily in Japan 6. 1980s U.S. recognizes the Deming approach and Taguchi philosophy to quality a. U.S. Industrial leaders begin to embrace the Deming philosophy of quality improvement (process control) and begin to use the statistical methods of Taguchi for improving process/product design II. Quality revolution of the 1980s A. Two philosophical changes needed 1. A change in the control model used: from product control to process control 2. Involvement of top management personnel in quality assurance B. Quality Control (product control - old philosophy)vs. Quality Assurance (process control - new philosophy) 1. Control of product vs. control of process 2. Productivity (profit) versus qualityvs. productivity (profit) and quality 3. "Police" activity vs. company-wide activity 4. Maintain quality vs. design-in and continually improve quality 5. Defect detection vs. defect prevention 6. Statistics for productivity and product rejection vs. statistics for process control and improvement 7. Company needs vs. customer needs III. Using statistical methods to assure quality A. Statistical process/product design 1. Used to develop a robust process/product design B. Statistical Process Control (SPC) 1. Used to detect and eliminate the sources of variation in the process that cannot be attributed to the routine operation of the process 2. How SPC is applied in food manufacturing a. Example: X-bar and Range Statistical Control Charts b. Chart parameters defined: (1) X-bar: average of n samples (X); process target value (2) X-bar-bar: process average obtained over time (3) UCL: upper control limit (approximately +3 standard deviations from the average) (4) LCL: lower control limit (approximately -3 standard deviations from the average) (5) Range value: highest X value minus lowest X value for each sampling event (6) R-bar: average process range difference 5. Global Hunger: Issues and Actions I. Background on global hunger A. A very serious problem, but not a new one 1. First written record of famine was in Egypt in 3500 BC B. Current status: Hunger map 2. Counting the hungry II. Defining global hunger issues A. Famine 1. A situation of extreme food scarcity, potentially leading to widespread starvation, often associated with crop failures (i.e., due to fungi, insects, or weather), war, or political unrest a. Example: 1845 fungus outbreak in Ireland 2. Finding enough food is usually not the principal problem 3. Getting the resources mobilized in time and overcoming political and logistical problems is usually the major obstacle to solving famine problems B. Short-term food shortage 1. Temporary shortages in food from decreased production due to inept agricultural policy, natural causes (e.g., low rainfall), or marketplace factors (e.g., dramatic price increases or decreases) C. Long-term undernutrition (or malnutrition) 1. Poverty is the common cause of undernutrition worldwide 2. Chronic food shortages that plague some regions of the world, due to many factors: a. Extreme imbalance in the food/population ratio in different regions of the world b. Depletion of natural resources c. High external country debt d. Poor infrastructure e. Lack of adequate agricultural practices and/or food processing and preservation technologies f. Social class structure g. Politics, war, and civil unrest 3. Types of malnutrition: a. Protein deficiency (called kwashiorkor) b. Chronic energy deficiency c. Protein-energy malnutrition (PEM) (called marasmus) d. Micronutrient (vitamin and/or mineral) deficiency (1) Most critical micronutrients missing from diets worldwide: iron, vitamin A, and iodide 4. Undernutrition diminishes both physical and mental capabilities and is complicated by infections and diseases 5. Most critical periods for undernutrition:pregnancy, infancy, childhood, and old age III. Is the global hunger problem becoming worse? A. Global facts to consider 1. Average daily energy supply is increasing: a. 1970: 2,440 kcals per person b. 2004: 3,000 to 3,500 kcals per person 2. Population control worldwide is still important B. The hunger situation in the U.S. C. The hunger situation in developing countries IV. Actions for combating global hunger A. What should we not do? 1. Feed the world through energy-intensive agricultural practices currently used in the U.S. a. When the entire food system is considered, 9 calories of fossil fuel are required to produce 1 calorie of food energy b. Feeding the entire world using energy-intensive agriculture as practiced in U.S. would quickly deplete global energy reserves (i.e., fossil fuel) 2. Direct food aid may be necessary, but is not a longterm solution B. What should we do? 1. Develop sustainable agricultural practices 2. Increase land allocation to agriculture 3. Increase food crop yields on that land 4. Increase efficiency of food utilization (what and how much we consume) and nutritional quality (specifically, protein quality) of existing and new food resources a. Most likely potential means of achieving this: Biotechnology You made it to the bottom! YAY [Show More]
Last updated: 2 years ago
Preview 1 out of 105 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 06, 2023
Number of pages
105
Written in
All
Additional information
This document has been written for:
Uploaded
Jan 06, 2023
Downloads
0
Views
227