Biology > STUDY GUIDE > BT 1111 Cancer Biology {CHEMOTHERAPY} study guide | Download To Score An A (All)
BT 1111 Cancer Biology {CHEMOTHERAPY} study guide | Download To Score An A
Document Content and Description Below
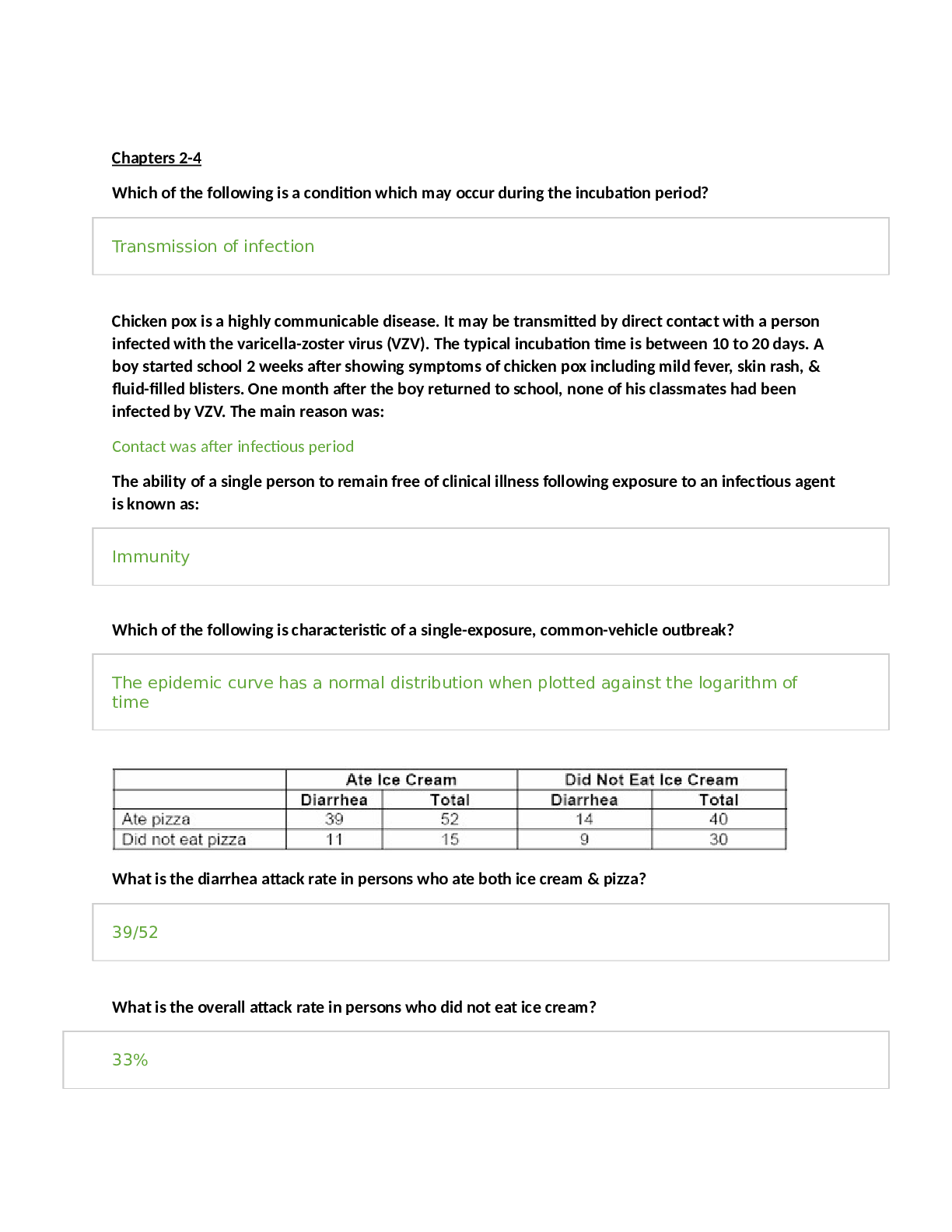
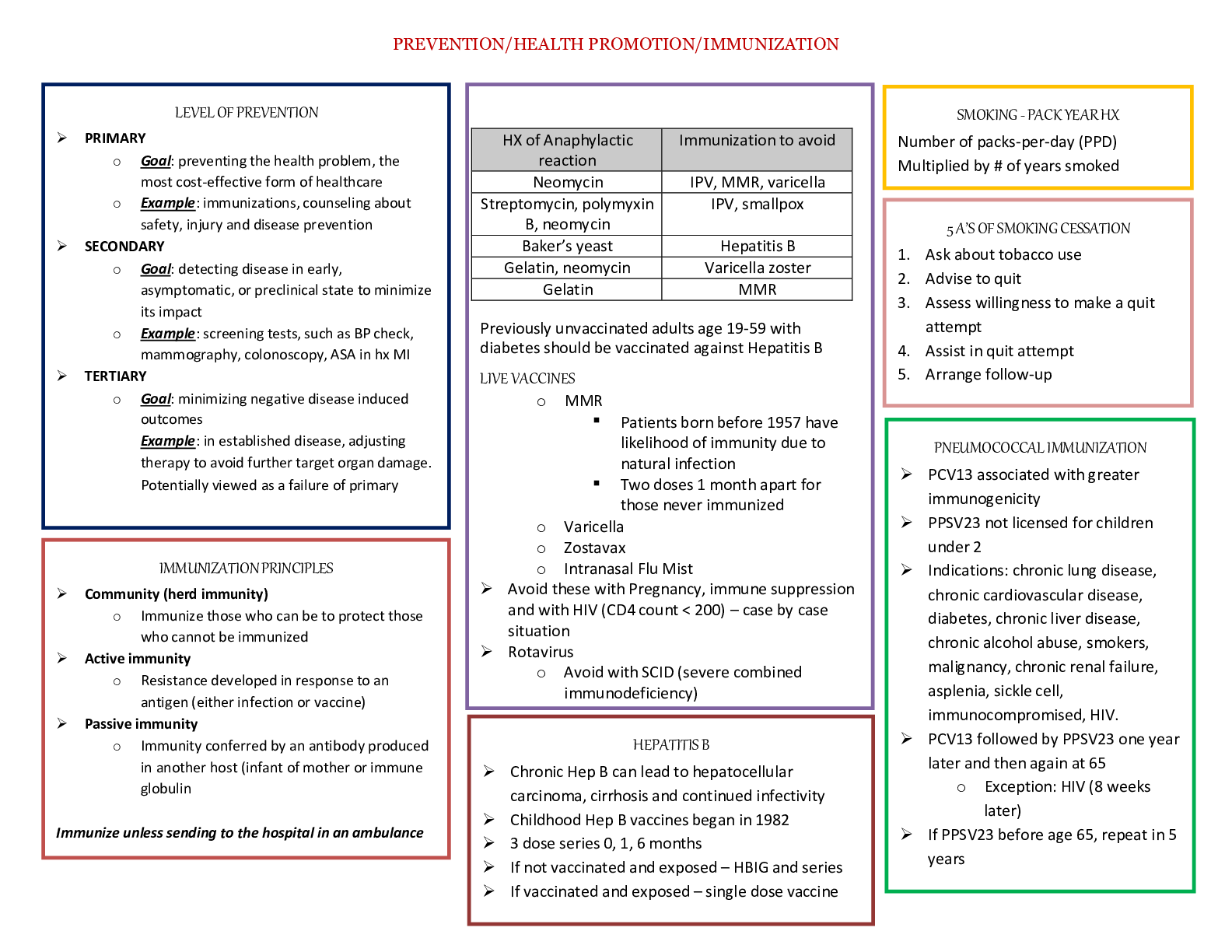
CHEMOTHERAPY Cell Cycle Specific Agents • Antimetabolites • Bleomycin • Podophyllin Alkaloids • Plant Alkaloids Cell Cycl... e Non-Specific Agents • Alkylating Agents • Antibiotics • Cisplatin • Nitrosoureas Alkylating Agents Nitrogen Mustards Ethylenimines Alkyl Sulfonates Nitrosoureas Cyclophosphamide Thiotepa Busulfan Carmustine Alkylating Agents Mechanism of Action • Alkylate within DNA at the N7 position of guanine • Resulting in miscoding through abnormal base- pairing with thymine or in depurination by excision of guanine residues, leading to strand breakage • Cross-linking of DNA and ring cleavage may also occur Mechanism of Action Toxicity • Bone marrow depression, with leukopenia and thrombocytopenia • Cyclophosphamide/Ifosfamide - hemorrhagic cystitis – Reduced by coadministration with MESNA • Cisplatin/Carboplatin - ototoxic and nephrotoxic – Nephrotoxicity reduced by chloride diuresis and hydration Therapeutic Uses • Used to treat a wide variety of hematologic and solid tumors • Thiotepa – ovarian cancer • Busulfan – chronic myeloid leukemia • Nitrosoureas - brain tumors • Streptozocin – insulin-secreting islet cell carcinoma of the pancreas Alkylating-Related Agents • Procarbazine • Dacarbazine • Altretamine • Cisplatin • Carboplatin Nitrogen Mustards • Cyclophosphamide • Ifosfamide • Mechlorethamine • Melphalan • Chlorambucil Cyclophosphamide Metabolism Nitrosoureas • Carmustine • Lomustine • Semustine • Streptozocin-naturally occuring sugar containing M.O.A.- cross-link through alkylation of DNA All cross the blood brain barrier Platinum Coordination Complexes These compounds alkylate N7 of guanine. They cause nephro- and ototoxicity. To counteract the effects of nephrotoxicity, give mannitol as an osmotic diuretic, or induce chloride diuresis with 0.1% NaCl. Antimetabolites Folic Acid Analogs Purine Analogs Pyrimidine Analogs Methotrexate Mercaptoguanine Fluorouracil Folic Acid Analogs • Methotrexate • Trimetrexate • Pemetrexed Folate • An essential dietary factor, from which THF cofactors are formed which provide single carbon groups for the synthesis of precursors of DNA and RNA • To function as a cofactor folate must be reduced by DHFR to THF Methotrexate Mechanism of Action ■ The enzyme DHFR is the 1º site of action ■ MTX prevents the formation of THF, causing an intracellular deficiency of folate coenzymes and accumulation of the toxic inhibitory substrate, DHF polyglutamate ■ The one carbon transfer reactions for purine and thymidylate synthesis cease, interrupting DNA and RNA synthesis Major Enzymatic Reactions Requiring Folates as Substrates* GAR GAR transformylase DHF b (1) AICAR transformylase AICAR IMP (3) 10- formylTHF Formate + THF e c AMP GMP (2) Methionine d dTMP 5,10-CH2THF 5-CH3THF a DNA *from Bowen dUMP Homocysteine Resistance Methotrexate Mechanism of Resistance 1. Decreased drug transport 2. Altered DHFR 3. Decreased polyglutamate formation 4. Increased levels of DHFR Methotrexate Therapeutic Uses • Methotrexate- psoriasis, rheumatoid arthritis, acute lymphoblastic leukemia, meningeal leukemia, choriocarcinoma, osteosarcoma, mycosis fungoides, Burkitt’s and non-Hodgkin’s lymphomas, cancers of the breast, head and neck, ovary, and bladder Methotrexate Toxicity • Bone marrow suppression – Rescue with leucovorin (folinic acid) • Nephrotoxic – give sodium bicarbonate to alkalinize the urine • Trimetrexate- Pneumocystis carinii pneumonia, metastatic colorectal carcinoma, head and neck carcinoma, pancreatic carcinoma, non-small cell carcinoma of the lung • Pemetrexed- Mesothelioma Purine Antagonists • Mercaptopurine • Thioguanine • Fludarabine Phosphate • Cladribine Mercaptopurine/Thioguanine • Must metabolized by HGPRT to the nucleotide form • This form inhibits numerous enzymes of purine nucleotide interconversion Fludarabine Phosphate • M.O.A.- phosphorylated intracellularly by deoxycytidine kinase to the triphosphate form • The metabolite inhibits DNA polymerase-α and ribonucleotide reductase • Induces apoptosis • Tx- non-Hodgkin’s lymphoma and chronic lymphocytic leukemia Cladribine • M.O.A. -phosphorylated by deoxycytidine kinase and is incorporated into DNA • Causes DNA strand breaks • Tx- hairy cell leukemia, chronic lymphocytic leukemia, and non-Hodgkin’s lymphoma Pyrimidine Antagonists • Fluorouracil - S-phase • Cytarabine • Gemcitabine • Capecitabine Figure 2. This figure illustrates the effects of MTX and 5-FU on the biochemical pathway for reduced folates. Mechanism of Action 5-FU • 5-FU inhibits thymidylate synthase therefore causing depletion of Thymidylate • 5-FU is incorporated into DNA • 5-FU inhibits RNA processing Activation of 5-FU Therapeutic Uses of 5-FU • Metastatic carcinomas of the breast and the GI tract • hepatoma • carcinomas of the ovary, cervix, urinary bladder, prostate, pancreas, and oropharyngeal areas • Combined with levamisole for Tx of colon cancer Cytarabine • It is activated to 5’ monophosphate (AraCMP) by deoxycytidine kinase • Through a series of reactions it forms the diphosphate (AraCDP) and triphosphate (AraCTP) nucleotides • Accumulation of AraCTP potently inhibits DNA synthesis • Inhibition of DNA synthesis is due to competitive (-) of polymerases and interference of chain elongation Cytarabine • It is a potent inducer of tumor cell differentiation • Fragmentation of DNA and evidence of apoptosis is noticed in treated cells • AraC is cell-cycle specific agent, it kills cells in the S-phase Cytarabine Mechanisms of Resistance • deficiency of deoxycytidine kinase • increased CTP synthase activity • increased cytidine deaminase activity • decreased affinity of DNA polymerase for AraCTP • decrease ability of the cell to transport AraC Therapeutic Uses • Induction of remissions in acute leukemia • Treats meningeal leukemia • Treatment of acute nonlymphocytic leukemia • In combination with anthracyclines or mitoxantrone it can treat non-Hodgkin’s lymphomas Toxicities • Nausea • acute myelosuppression • stomatitis • alopecia • Gemcitabine is S-phase specific • it is a deoxycytidine antimetabolite • it undergoes intracellular conversion to gemcitabine monophosphate via the enzyme deoxycytidine kinase • it is subsequently phosphorylated to gemcitabine diphosphate and gemcitabine triphosphate • Gemcitabine triphosphate competes with deoxycytidine triphosphate (dCTP) for incorporation into DNA strands • do to an addition of a base pair before DNA polymerase is stopped, Gemcitabine inhibits both DNA replication and repair • Gemcitabine-induced cell death has characteristics of apoptosis Gemcitabine Therapeutic Uses • Gemcitabine treats a variety of solid tumors • very effective in the treatment of pancreatic cancer • small cell lung cancer • carcinoma of the bladder, breast, kidney, ovary, and head and neck Plant Alkaloids Vinca Alkaloids Podophyllotoxins Camptothecins Taxanes Vinblastine Etoposide Topotecan Paclitaxel Vinca Alkaloids • Vinblastine • Vincristine • Vinorelbine Vinca Alkaloids Inhibit microtubules (spindle), causing metaphase cell arrest in M phase. 3 3 Vinca Alkaloids Mechanism of Action • Binds to the microtubular protein tubulin in a dimeric form • The drug-tubulin complex adds to the forming end of the microtubules to terminate assembly • Depolymerization of the microtubules occurs • Resulting in mitotic arrest at metaphase, dissolution of the mitotic spindle, and interference with chromosome segregation • CCS agents- M phase Toxicity • Nausea • Vomiting • Marrow depression • Alopecia Therapeutic Uses • Systemic Hodgkin’s disease • Lymphomas Toxicity • Muscle weakness • Peripheral neuritis Therapeutic Uses • With prednisone for remission of Acute Leukemia • Granulocytopenia Toxicity Therapeutic Uses • non-small cell lung cancer Podophyllotoxins • Etoposide (VP-16) • Teniposide (VM-26) • Semi-synthetic derivatives of podophyllotoxin extracted from the root of the mayapple Mechanism of Action • Blocks cells in the late S-G2 phase of the cell cycle through inhibition of topoisomerase II • Resulting in DNA damage through strand breakage induced by the formation of a ternary complex of drug, DNA, and enzyme Toxicity • Nausea • Vomiting • Alopecia • Hematopoietic and lymphoid toxicity Therapeutic Uses • Monocytic Leukemia • Testicular cancer • Oat cell carcinoma of the lung Camptothecins • Topotecan • Irinotecan Mechanism of Action • Interfere with the activity of Topoisomerase I • Resulting in DNA damage • Irinotecan- a prodrug that is metabolized to an active Top I inhibitor, SN-38 Toxicity • Topotecan – Neutropenia, thrombocytopenia, anemia • Irinotecan – Severe diarrhea, myelosuppression Camptothecins Therapeutic Uses • Topotecan- metastatic ovarian cancer (cisplatin-resistant) • Irinotecan- colon and rectal cancer Taxanes • Paclitaxel (Taxol) • Docetaxel • Alkaloid esters derived from the Western and European Yew Mechanism of Action • Mitotic “spindle poison” through the enhancement of tubulin polymerization • Paclitaxel Toxicity – Neutropenia, thrombocytopenia – Peripheral neuropathy • Docetaxel – Bone marrow suppression – Neurotoxicity – Fluid retention Therapeutic Uses • Paclitaxel- ovarian and advanced breast cancer • Docetaxel- advanced breast cancer Antibiotics • Anthracyclines- Doxorubicin & Daunorubicin • Dactinomycin • Plicamycin • Mitomycin • Bleomycin Anthracyclines • Doxorubicin • Daunorubicin Mechanism of Action • High-affinity binding to DNA through intercalation, resulting in blockade of DNA and RNA synthesis • DNA strand scission via effects on Top II • Binding to membranes altering fluidity • Generation of the semiquinone free radical and oxygen radicals Toxicity • Bone marrow depression • Total alopecia • Cardiac toxicity Therapeutic Uses • Doxorubicin- carcinomas of the breast, endometrium, ovary, testicle, thyroid, and lung, Ewing’s sarcoma, and osteosarcoma • Daunorubicin- acute leukemia Dactinomycin Mechanism of Action • Binds to double stranded DNA through intercalation between adjacent guanine- cytosine base pairs • Inhibits all forms of DNA-dependent RNA synthesis Toxicity • Bone marrow depression • Oral ulcers • Skin eruptions • Immunosuppression Therapeutic Uses • Wilms’ tumors • Gestational choriocarinoma with MTX Mechanism of Action • Binds to DNA through an antibiotic-Mg2+ complex • This interaction interrupts DNA-directed RNA synthesis Toxicity • Hypocalcemia • Bleeding disorders • Liver toxicity Therapeutic Uses • Testicular cancer • Hypercalcemia Mechanism of Action • Bioreductive alkylating agent that undergoes metabolic reductive activation through an enzyme-mediated reduction to generate an alkylating agent that cross-links DNA Toxicity • Severe myelosuppression • Renal toxicity • Interstitial pneumonitis Therapeutic Uses • Squamous cell carcinoma of the cervix • Adenocarcinomas of the stomach, pancreas, and lung • 2nd line in metastatic colon cancer Bleomycin • Acts through binding to DNA, which results in single and double strand breaks following free radical formation and inhibition of DNA synthesis • The DNA fragmentation is due to oxidation of a DNA-bleomycin-Fe(II) complex and leads to chromosomal aberrations • CCS drug that causes accumulation of cells in G2 Toxicity • Lethal anaphylactoid reactions • Blistering • Pulmonary fibrosis Therapeutic Uses • Testicular cancer • Squamous cell carcinomas of the head and neck, cervix, skin, penis, and rectum • Lymphomas • Intracavitary therapy in ovarian and breast cancers Hormonal Agents Estrogen & Androgen Inhibitors Gonadotropin-Releasing Hormone Agonists Aromatase Inhibitors Tamoxifen Leuprolide Aminogluthethimide Anti-Estrogens • Tamoxifen (SERMs) • Raloxifene (SERMs) • Faslodex Tamoxifen • Selective estrogen receptor modulator (SERM), have both estrogenic and antiestrogenic effects on various tissues • Binds to estrogen receptors (ER) and induces conformational changes in the receptor • Has antiestrogenic effects on breast tissue. • The ability to produce both estrogenic and antiestrogenic affects is most likely due to the interaction with other coactivators or corepressors in the tissue and the binding with different estrogen receptors, ER? and ER? • Subsequent to tamoxifen ER binding, the expression of estrogen dependent genes is blocked or altered • Resulting in decreased estrogen response. • Most of tamoxifen’s affects occur in the G1 phase of the cell cycle Toxicity • Hot flashes • Fluid retention • nausea Therapeutic Uses • Tamoxifen can be used as primary therapy for metastatic breast cancer in both men and postmenopausal women • Patients with estrogen-receptor (ER) positive tumors are more likely to respond to tamoxifen therapy, while the use of tamoxifen in women with ER negative tumors is still investigational • When used prophylatically, tamoxifen has been shown to decrease the incidence of breast cancer in women who are at high risk for developing the disease • Flutamide Anti-Androgen – Antagonizes androgenic effects – approved for the treatment of prostate cancer Agonists • Leuprolide • Goserelin Mechanism of Action • Agents act as GnRH agonist, with paradoxic effects on the pituitary • Initially stimulating the release of FSH and LH, followed by inhibition of the release of these hormones • Resulting in reduced testicular androgen synthesis Toxicity • Gynecomastia • Edema • thromboembolism Therapeutic Uses • Metastatic carcinoma of the prostate • Hormone receptor-positive breast cancer Aromatase Inhibitors • Aminogluthethimide • Anastrozole Aminogluthethimide Mechanism of Action • Inhibitor of adrenal steroid synthesis at the first step, conversion of cholesterol of pregnenolone • Inhibits the extra-adrenal synthesis of estrone and estradiol • Inhibits the enzyme aromatase that converts androstenedione to estrone Aminogluthethimide Toxicity • Dizziness • Lethargy • Visual blurring • Rash Therapeutic Uses • ER- and PR-positive metastatic breast cancer Anastrozole • A new selective nonsteroidal inhibitor of aromatase • Treats advanced estrogen and progesterone receptor positive breast cancer that is no longer responsive to tamoxifen Miscellaneous AntiCancer Agents • Asparaginase • Hydroxurea • Mitoxantrone • Mitotane • Retinoic Acid Derivatives • Amifostine • An enzyme isolated from bacteria • Causes catabolic depletion of serum asparagine to aspartic acid and ammonia • Resulting in reduced blood glutamine levels and inhibition of protein synthesis • Neoplastic cells require external source of asparagine • Treats childhood acute leukemia • Can cause anaphylactic shock • An analog of urea • Inhibits the enzyme ribonucleotide reductase • Resulting in the depletion of deoxynucleoside triphosphate pools • Thereby inhibiting DNA synthesis • S-phase specific agent • Treats melanoma and chronic myelogenous leukemia • Structure resembles the anthracyclines • Binds to DNA to produce strand breakage • Inhibits DNA and RNA synthesis • Treats pediatric and adult acute myelogenous leukemia, non-Hodgkin’s lymphomas, and breast cancer • Causes cardiac toxicity Mechanisms & Actions of Useful Chemotherapeutic Drugs in Neoplastic Disease [Show More]
Last updated: 2 years ago
Preview 1 out of 109 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jun 30, 2021
Number of pages
109
Written in
Additional information
This document has been written for:
Uploaded
Jun 30, 2021
Downloads
0
Views
50