Chemistry > Lab Report > Lab Report >7.03 Calorimetry Lab Report_ Parkersburg South High School. Plus Q&A (All)
Lab Report >7.03 Calorimetry Lab Report_ Parkersburg South High School. Plus Q&A
Document Content and Description Below
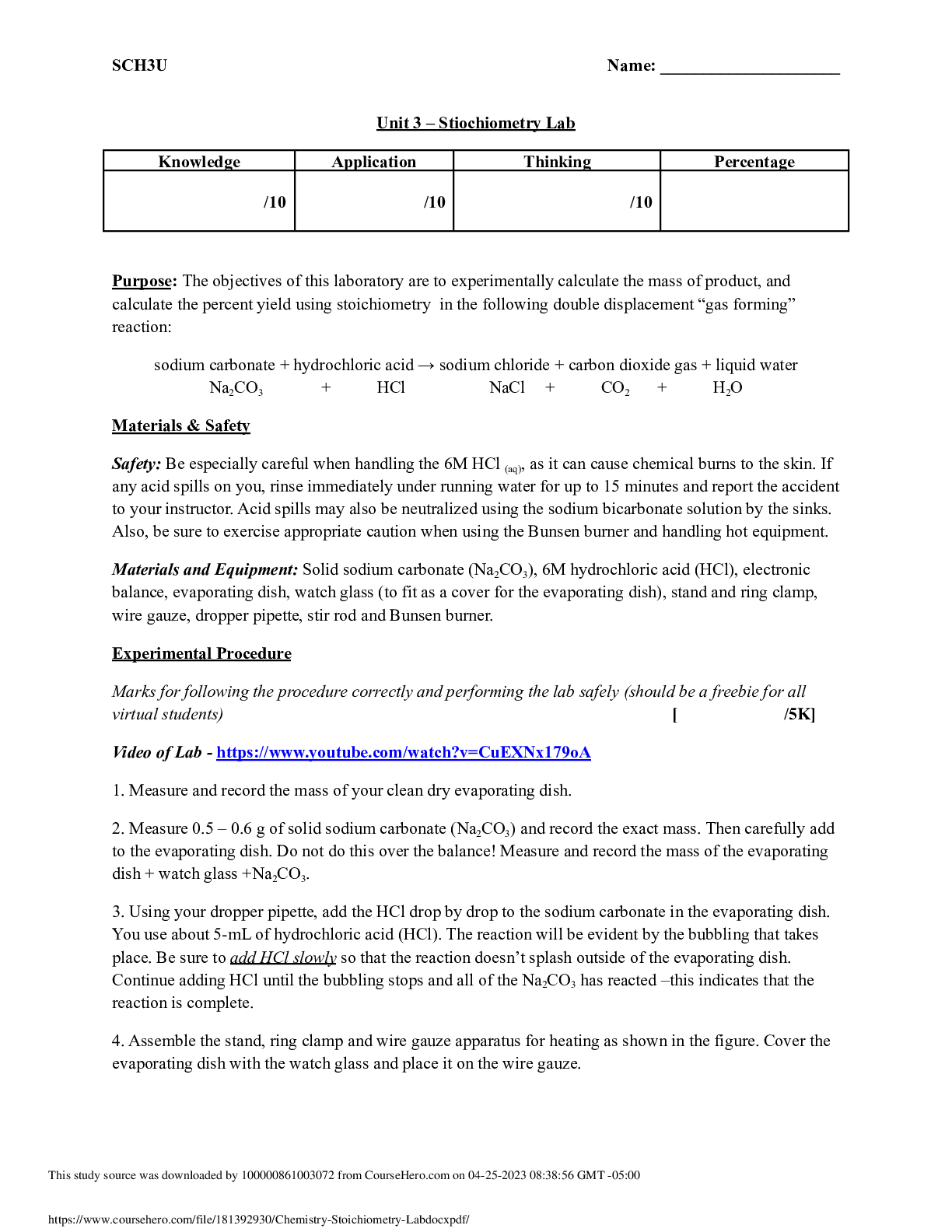
7.03 Calorimetry Lab Report AP Chemistry 2/15/19 Drawing of my calorimeter Data Water Before After Hot 49OC 36OC Cold 24OC 36OC Analysis Questions or Calculations (1 – 4) 1. Calculate the en ... thalpy change of the cold water using the equation qhot = mhotcΔThot. Assume that the density of water is exactly 1 g/mL. Is this an endothermic or exothermic process? Explain. The enthalpy change of the cold water is exothermic because the hot water lost its heat to the cold water. 2. Calculate the enthalpy change of the hot water using the equation qcold = mcoldcΔTcold. Assume that the density of water is exactly 1 g/mL. Is this an endothermic or exothermic process? Explain. The enthalpy change of the hot water is endothermic because the cold water lost its cold because it absorbed heat from the hot water. 3. These amounts are not equal because the calorimeter (the coffee cups) absorbs some of the thermal energy transferred by the hot water. Thus under the real conditions observed in the laboratory the law of conservation of energy equation becomes qhot = – (qcold + qcal), where qcal is the enthalpy change of the calorimeter. Use this equation to calculate the enthalpy change of the calorimeter. 4. The calorimeter constant, C, is the heat absorbed by the calorimeter per degree of temperature change, C = qcal/ΔTcal. Assuming the starting temperature of the calorimeter is the same as that of the cold water, calculate the calorimeter constant in units of joules per degree Celsius. [Show More]
Last updated: 2 years ago
Preview 1 out of 2 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$4.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 23, 2023
Number of pages
2
Written in
All
Additional information
This document has been written for:
Uploaded
Jan 23, 2023
Downloads
0
Views
174











.png)





.png)



