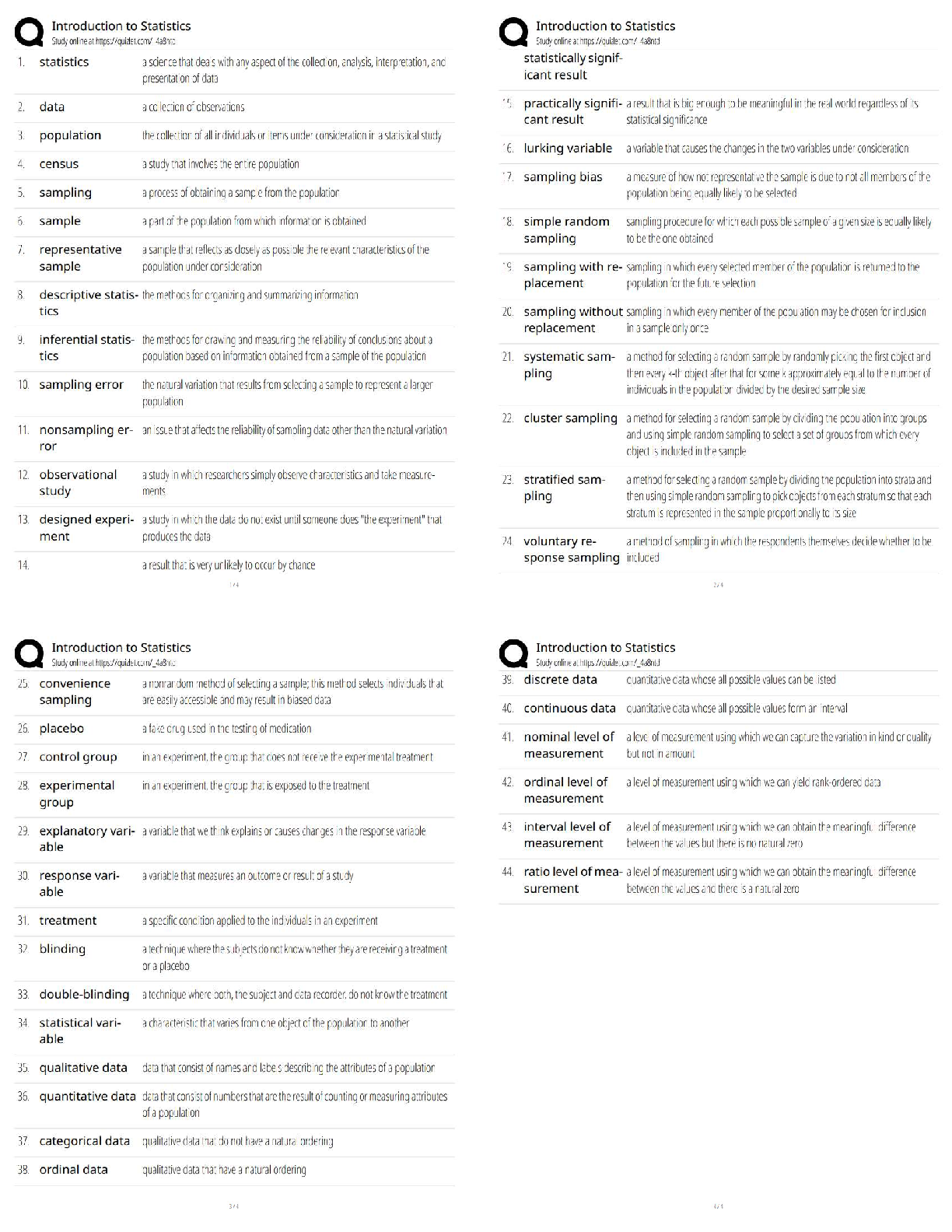
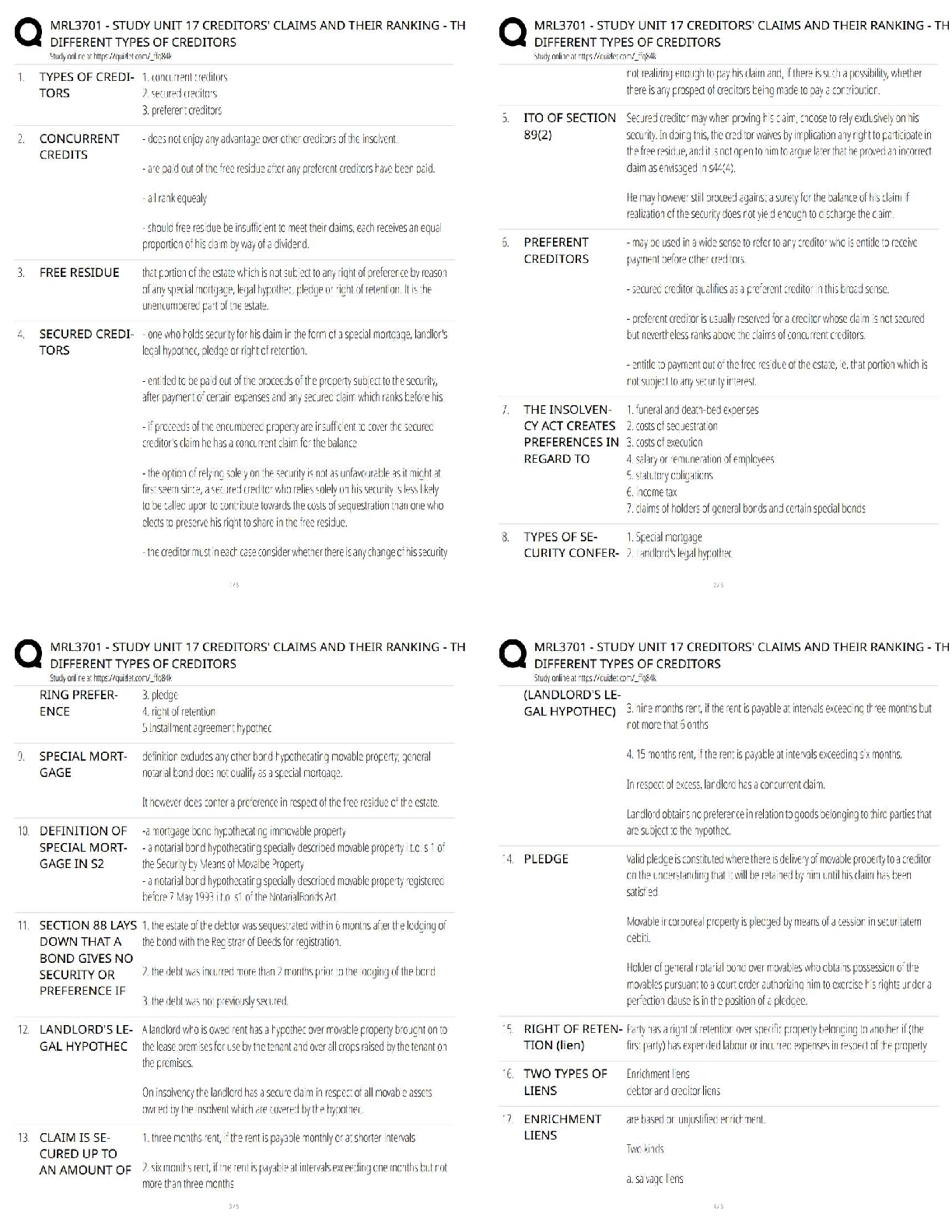
MIDTERM: MATSE 201 Exam
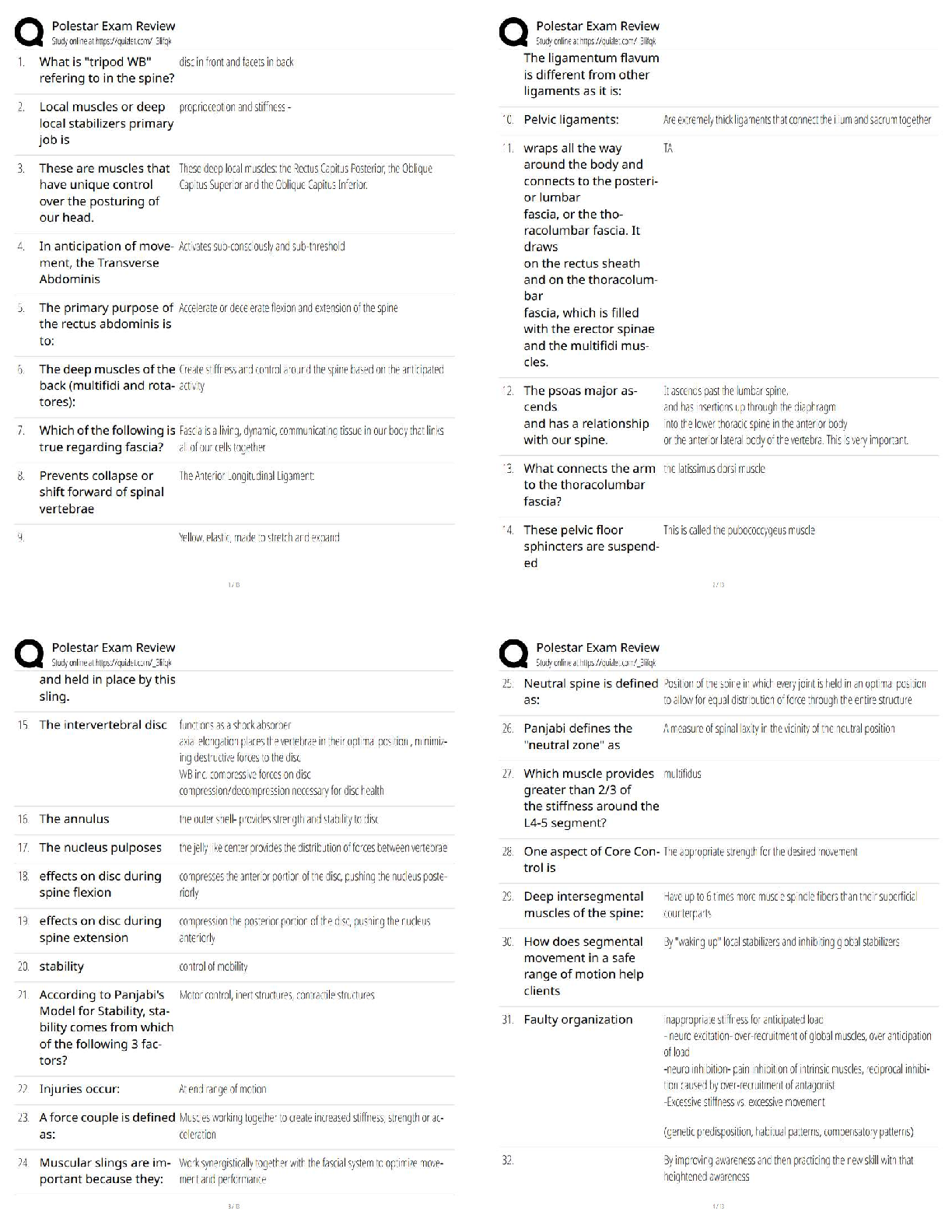
1. Which is true regarding the seven crystal systems?
A) they completely define a crystal structure
B) they describe the shape of the unit cell.
C) there are 7 shapes that can complet
...
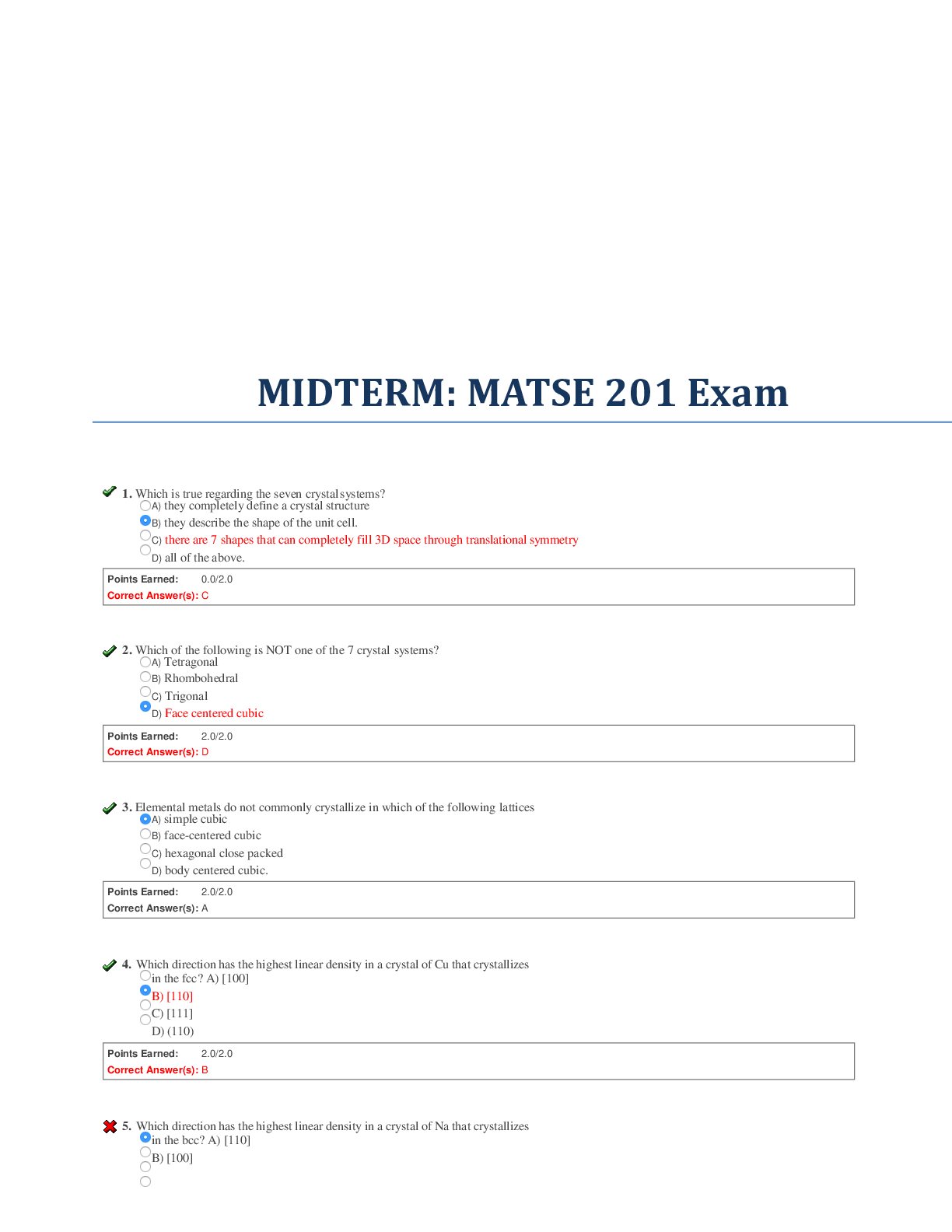
MIDTERM: MATSE 201 Exam
1. Which is true regarding the seven crystal systems?
A) they completely define a crystal structure
B) they describe the shape of the unit cell.
C) there are 7 shapes that can completely fill 3D space through translational symmetry
D) all of the above.
2. Which of the following is NOT one of the 7 crystal systems?
A) Tetragonal
B) Rhombohedral
C) Trigonal
D) Face centered cubic
3. Elemental metals do not commonly crystallize in which of the following lattices
A) simple cubic
B) facecentered cubic
C) hexagonal close packed
D) body centered cubic.
4. Which direction has the highest linear density in a crystal of Cu that crystallizes in the fcc? A) [100]
B) [110]
C) [111]
D) (110)
5. Which direction has the highest linear density in a crystal of Na that crystallizes in the bcc? A) [110]
B) [100]
C) [111]
D) (111)
6. In a BCC metal, the plane with the highest planer density is: A) (100)
B) (110)
C) (111)
D) (200)
7. The closest packed plane in a metal FCC structure is: A) (100)
B) (110)
C) (111)
D) (200)
8. Which is TRUE about Bragg’s Law?
A) It defines conditions necessary for diffraction in primitive cells
B) It is insufficient to predict diffraction in nonprimitive cells
C) It relates the the path length difference between to diffracted beams to the lattice dspacing
D) All of the above
9. Which plane will show a peak on a diffraction pattern for alphaFe (metal bcc structure)? A) (100)
B) (220)
C) (210)
D) (221)
10. Which of the following statements about vacancies is true?
A) vacancies are always present in materials
B) as temperature increases, the number of vacancies will increase
C) as temperature decreases, the number of vacancies will increase
D) vacancies are considered linear defects
11. The HumeRothery rules give the conditions to be met for
A) Mixing between two elements
B) Assignment of coordination number
C) The existence of an impurity
D) Complete solid solubility
12.
Which of the following describe the defect represented by AlMg•
A) Aluminum vacancy on a magnesium site
B) Magnesium vacancy on an aluminum site
C) Aluminum substitution on a magnesium site
D) Magnesium substitution on an aluminum site
13. Which of the following could be used to maintain electroneutrality for the substitution of an aluminum atom into a magnesium lattice point?
A) For every two aluminum substitutions, create one oxygen vacancy
B) For every two aluminum substitutions, create a magnesium vacancy
C) Electroneutrality is maintained for this substitution
D) For every two aluminum substitutions, create a magnesium interstitial
14. Which is true regarding dislocations in crystals?
A) they are categorized as being twodimensional defects
B) they occur only in metals
C) they can be described quantitatively by their Burger’s vector
D) cannot be readily detected because they are too small.
15. Twin boundaries, grain boundaries, and stacking faults all represent which kind of defect?
A) Types of dislocations
B) Two dimensional defects
C) Linear defects
D) Point defects
16. Which of the following statements describes Fick's first law?
A) the diffusivity is proportional to the concentration gradient
B) the net mass flux is proportional to the diffusivity
C) the flux is described by the error function
D) the diffusivity is thermally activated
17. Diffusion in a solid
A) is facilitated by point defects such as vacancies
B) is a function of temperature
C) requires a concentration gradient,
D) all of the above.
18. A condition of nonsteady state diffusion (i.e. the net flux is varying with time) can be described by
A) Fick’s Second law,
B) the Avrami equation
C) an Arrhenius relationship
D) none of the above.
19. Diffusivity has units of
A) mass/time2
B) length2/time
C) mass/length2•time
D) masslength/time
20. Which of the following statements about diffusion is TRUE?
A) As the bond strength is increased, the diffusion coefficient increases
B) The smaller the diffusing species the smaller the diffusivity
C) As atomic packing factor increases, diffusivity increases
D) As bond energy increases, the activation energy for diffusion increases
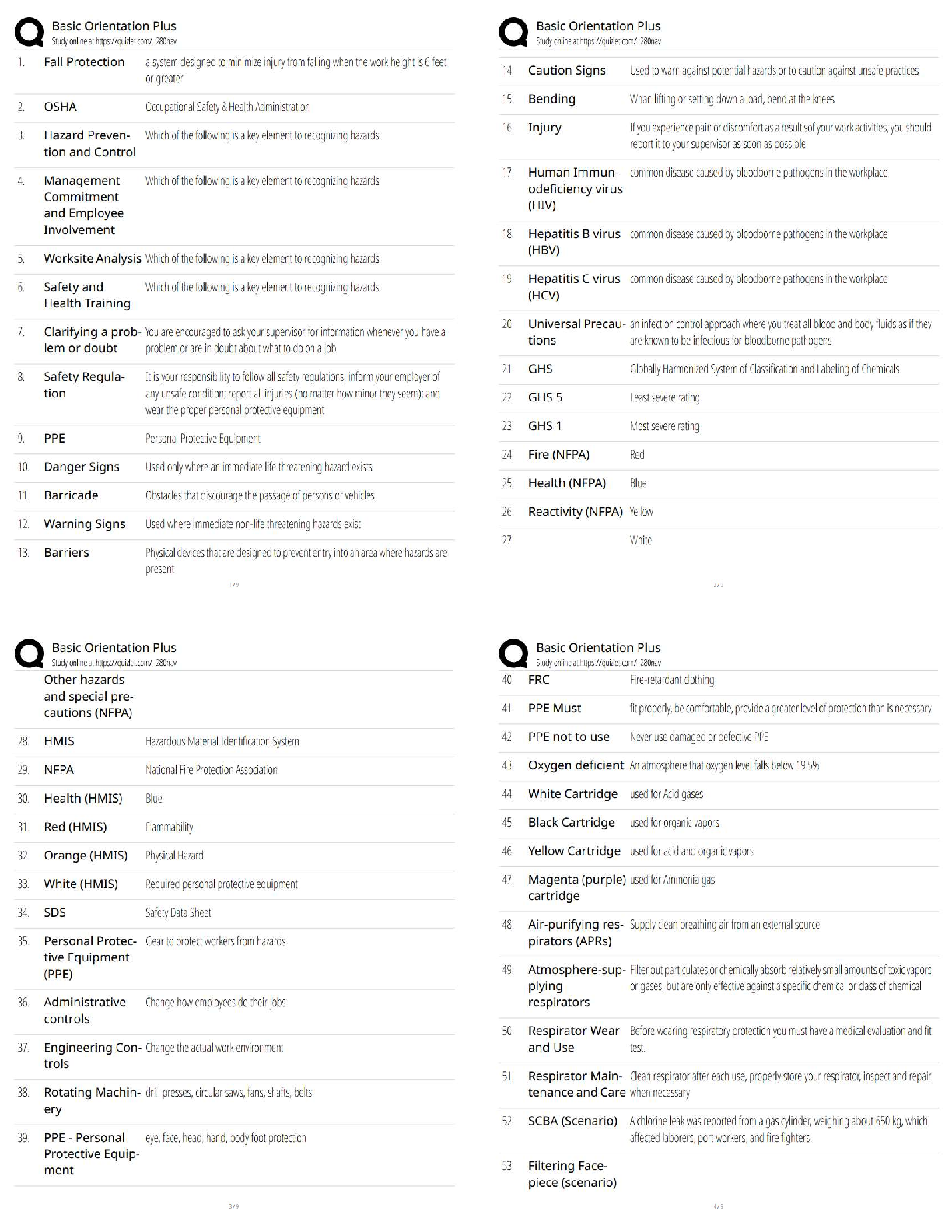
21.
NaCl crystallizes in the Rocksalt structure illustrated above. (the figure shows multiple unit cells together). Its xray diffraction pattern for lamda = 0.154 nm is also shown. The table attached shows relevant data for atoms and ions which you may find useful to answer the questions, 2124, below.
21. Consider NaCl as well as the compounds MgO, MgS, and KBr which also crystal in the same Rocksalt structure. Which shows the ranking order these compounds from lowest to highest melting point?
A) MgS < MgO < KBr < NaCl
B) MgO < MgS < KBr < NaCl
C) NaCl < KBr < MgS < MgO
D) NaCl < KBr < MgO < MgS
22.
Refer to the picture in question 21. The peak for the (220) takes place at a 2theta value of 45 degrees. Calculate the lattice parameter for NaCl. The value of the lattice parameter is
A) 0.201 nm
B) 0.569 nm
C) 0.109 nm
D) 0.308 nm
23. Refer to the figure in question 21. The calculated the theoretical density of NaCl is A) 13.29 g/cm3
B) 1.05 g/cm3
C) 2.11 g/cm3
D) 0.527 g/cm3
24.
Refer to the figure in question 21. For a similar XRD pattern for MgS (with lamda=0.154 nm) at what diffraction angle (2theta) would you expect the first peak to appear?
A) 30.2 degrees
B) 15.1 degrees
C) 17.9 degrees
D) 35.8 degrees
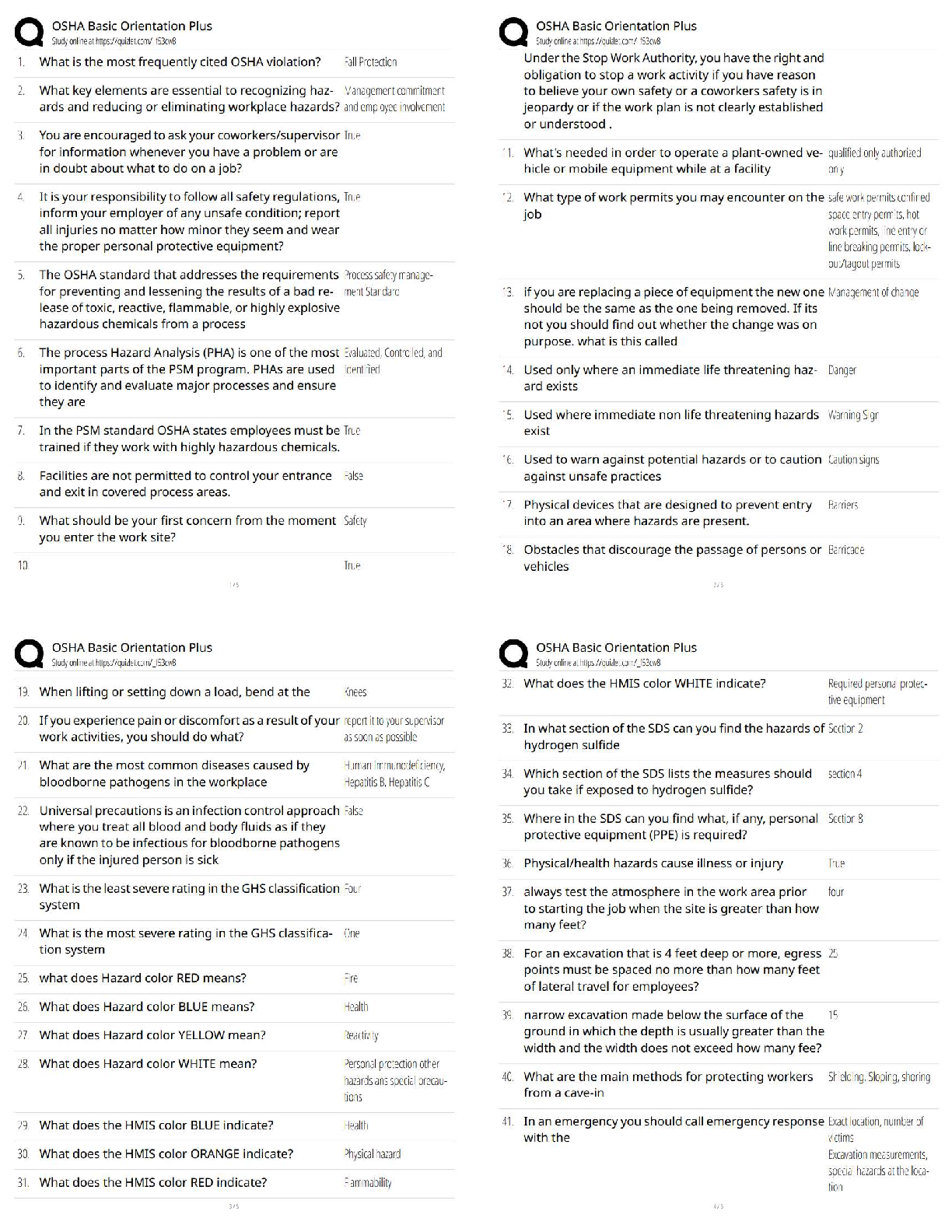
25.
Refer to figures (i) – (ii) to answer questions 25 – 26 about the specific directions and planes depicted below using proper Miller (or MillerBravais) indices.
25. Which choice indicates the three directions shown in Figure i?
A) (101), (111), (010)
B) [101], [111], [010]
C) [110], [010], [011]
D) None of the above
26. Which choice indicates the plane shown in Figure ii, in question 25? A) [101]
B) (110)
C) [110]
D) (101)
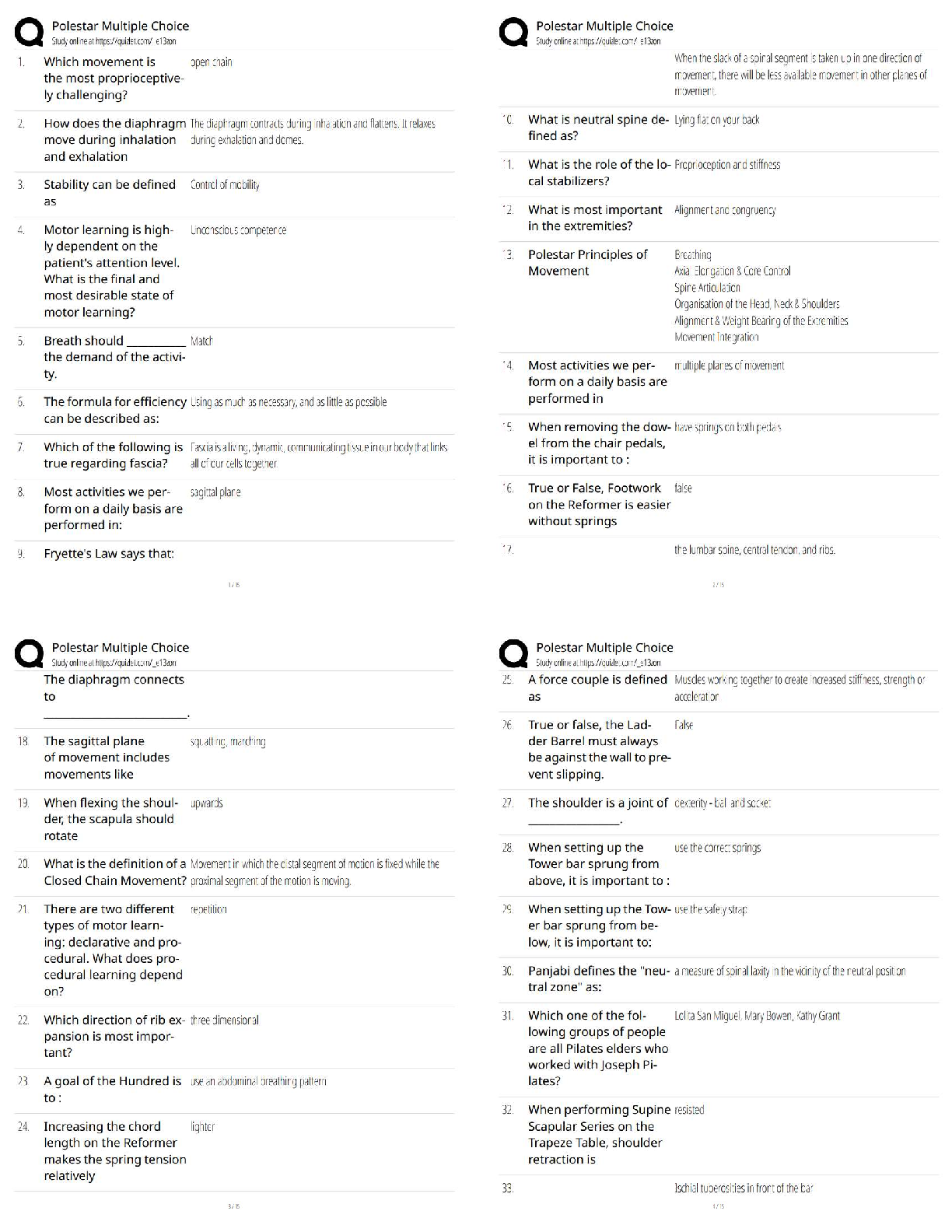
27.
Using a MgCl2 crystal as an example, Which of the following choices describes a Frenkel defect reaction in this material?
A) Null → VMg″ + VCl•
B) MgCl2 → VMg″ + 2Cli•
C) MgCl2 → VMg″ + Mgi•• +2Cl x
D) Null → VMg″ + 2VCl•
28.
Using a MgCl2 crystal as an example, If the fraction of intrinsically vacant Mg sites in this crystal is 2.3 x1013 at 500°C and the activation energy for this vacancy formation is Ev = 2.3 eV, calculate the fraction of Mg vacancies expected to exist at 800°C.
A) 3.6 x109
B) 1.1 x104
C) 1.4 x1017
D) 4.6 x1022
29.
Using a MgCl2 crystal as an example, If the compound was MgBr2, how would you expect the slope of the curve to change in a plot of ln (fraction of intrinsically vacant Mg sites) as a function of 1/T?
A) Slope of curve would become positive
B) Slope of the curve would decrease
C) Slope of the curve would increase
D) Slope would stay the same
30.
Using a hydrocarbon gas to carburize the surface of a steel, we obtain a surface carbon content of 1.0 wt%. The initial carbon content of the steel is 0.2 wt%. How long would take at 1000˚C to obtain a carbon concentration of 0.6 wt% at a distance 1mm from the surface? Given: Do = 20 x 106 m2/S; Q = 142 kJ/mol
A) 1.52 x105 hours
B) 1.02 x107 hours
C) 15.2 hours
D) 10.2 hours
[Show More]







.png)

.png)


















.png)
.png)
.png)