Chemistry > QUESTIONS & ANSWERS > BCHE 5180/6180 Exam Final. 100% Answers Provided (All)
BCHE 5180/6180 Exam Final. 100% Answers Provided
Document Content and Description Below
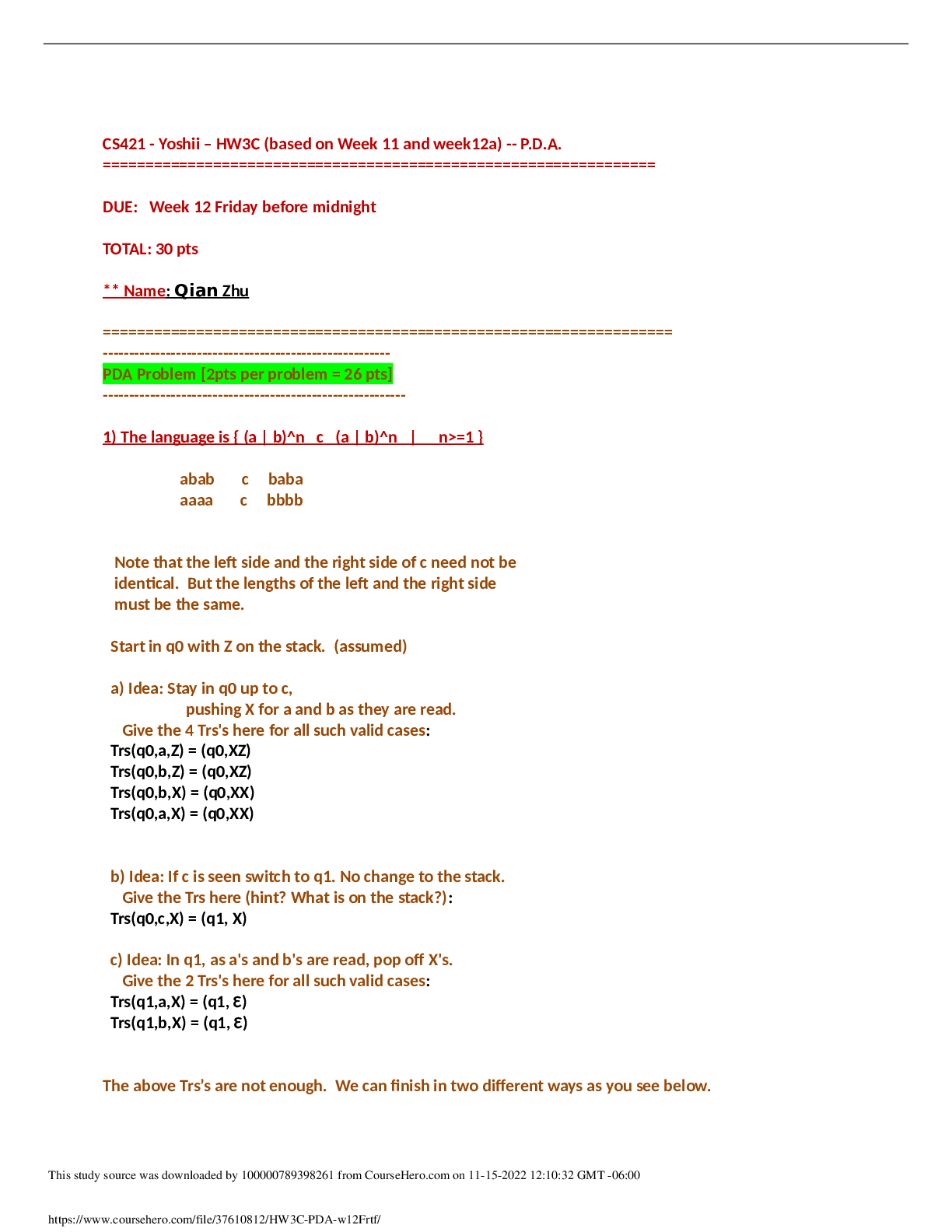
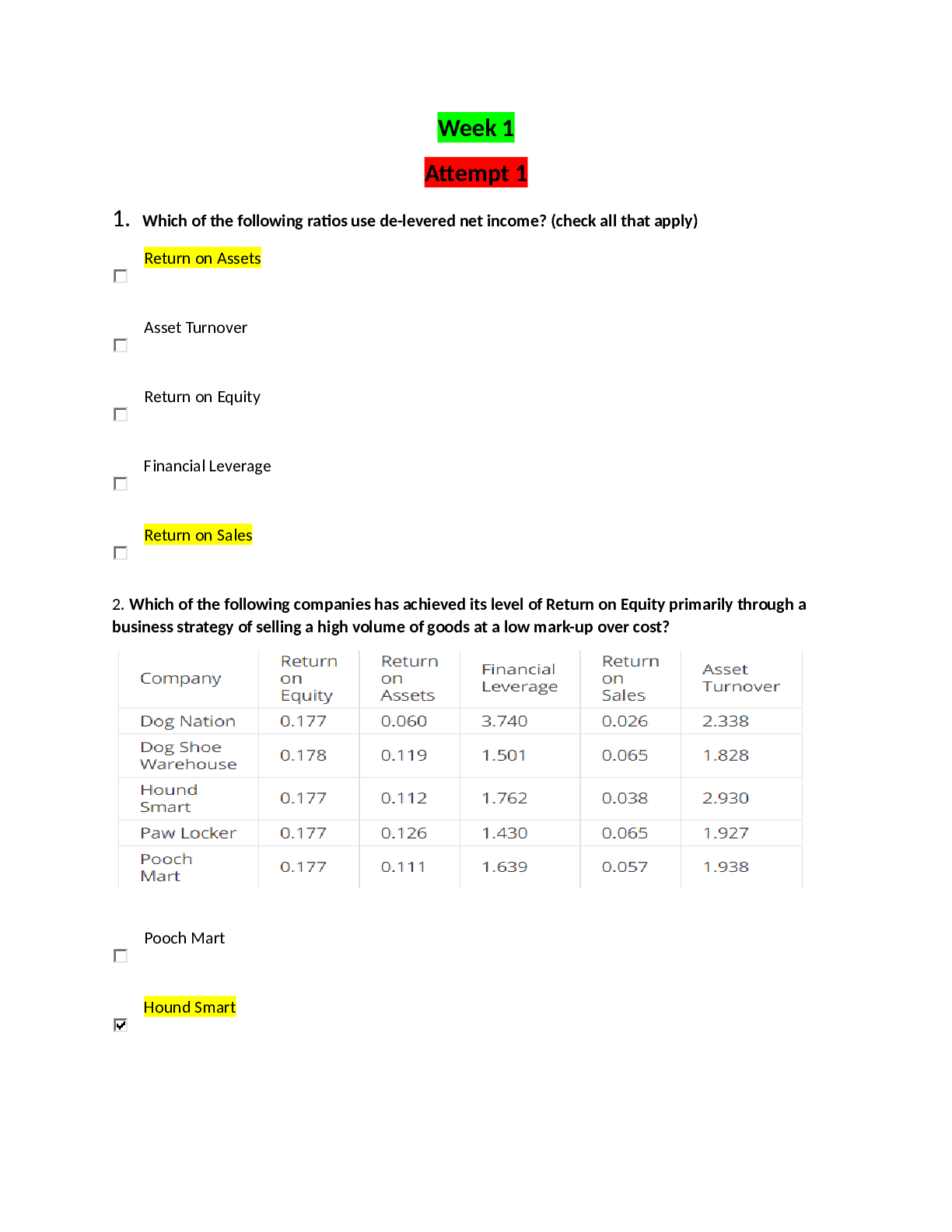
1. Give the general Henderson-Hasselbalch equation and sketch the plot it describes (Ph against amount of NaOH added to a weak acid). On your curve label the pKa for the weak acid, and indicate the re... gion in which the buffering capacity of the system is greatest. 2. Name and briefly define four types of noncovalent interactions that occur between biological molecules. 3. Draw the structure of alanine, leucine, isoleucine, tyrosine, lysine and histidine (At pH 7.0). Give also the three-letter and the one-letter codes. Indicate the pKa of the side groups. (Draw 1 amino acid completely, draw only the R group for the other amino acids). 4. Explain the differences between common and uncommon amino acids 5. Given below is the structure of the polypeptide Glu-Gly-Leu-Ser-Leu-Ser-Lys. 6. What is the charge of the peptide at pH 3.0, pH 8.0, and pH 12? 7. What is the pI of the peptide? 8. A student has purified a protein. The table below describes the followed procedure: The student also prepared an SDS-PAGE gel: 9. Explain the SDS-PAGE technique 10. Can you use this information to tell whether the purification was successful? Note that there are different answers possible. What would be the next logical step to discern between these possibilities? 11. Describe the principle of ion-exchange chromatography 12. Explain in detail the different steps of sequencing a peptide/protein and their purpose 13. What can we learn from sequence alignments 14. A biochemist purified a polypeptide containing 8 amino acids Amino acid analysis showed the following composition: 15. Ala, Glu, Leu, (Lys)2, Met, Tyr, Val The native peptide was incubated with 1-fluoro-2,4-dinitrobenzene (FDNB) and then hydrolyzed; 2,4-dinitrophenylglutamate was identified by HPLC. Incubation of the native peptide with trypsin gave a tripeptide, a tetrapeptide and a single Ala. Incubation with FDNB found a Glu residue for the tripeptide, a Met residue for the tetra peptide. When the native peptide was exposed to cyanogen bromide (CNBr), two tetrapeptides were obtained. Incubation with FDNB found both 2,4-dinitrophenylglutamate and 2,4 dinitrophenyltyrosine. The amino acid composition of one of the pentapeptides was Ala, Lys, Tyr, Val. What is the sequence of the polypeptide? 16. The picture on the right shows the ribbon model of a protein. 17. Explain what the ribbon represents. The ribbon represents the position of the six-atom that lye in the same plane as the peptide bond: 18. What do you see? What is the main type of secondary structure present in this figure? The long stretches of ribbon indicate that the main type of secondary structure is of the -sheet type. (The sheets are organized as a -barrel). The remaining structure has a ”random” structure. No α-helices are present. 19. Explain what properties give this type of secondary structure its stability. 20. Can you tell if this is a globular, fibrous or membrane protein? Explain your answer. 21. Why are glycine and proline often found within a β turn? 22. Explain the differences between subunit, domain and fold. Explain the difference between protein families and protein super families [Show More]
Last updated: 2 years ago
Preview 1 out of 10 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$13.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 15, 2020
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
Mar 15, 2020
Downloads
0
Views
69




.png)
.png)
.png)
.png)
.png)
.png)
.png)

