Biology > QUESTIONS & ANSWERS > Chapter 05: Performance Improvement in the Microbiology Laboratory. All Answered (All)
Chapter 05: Performance Improvement in the Microbiology Laboratory. All Answered
Document Content and Description Below
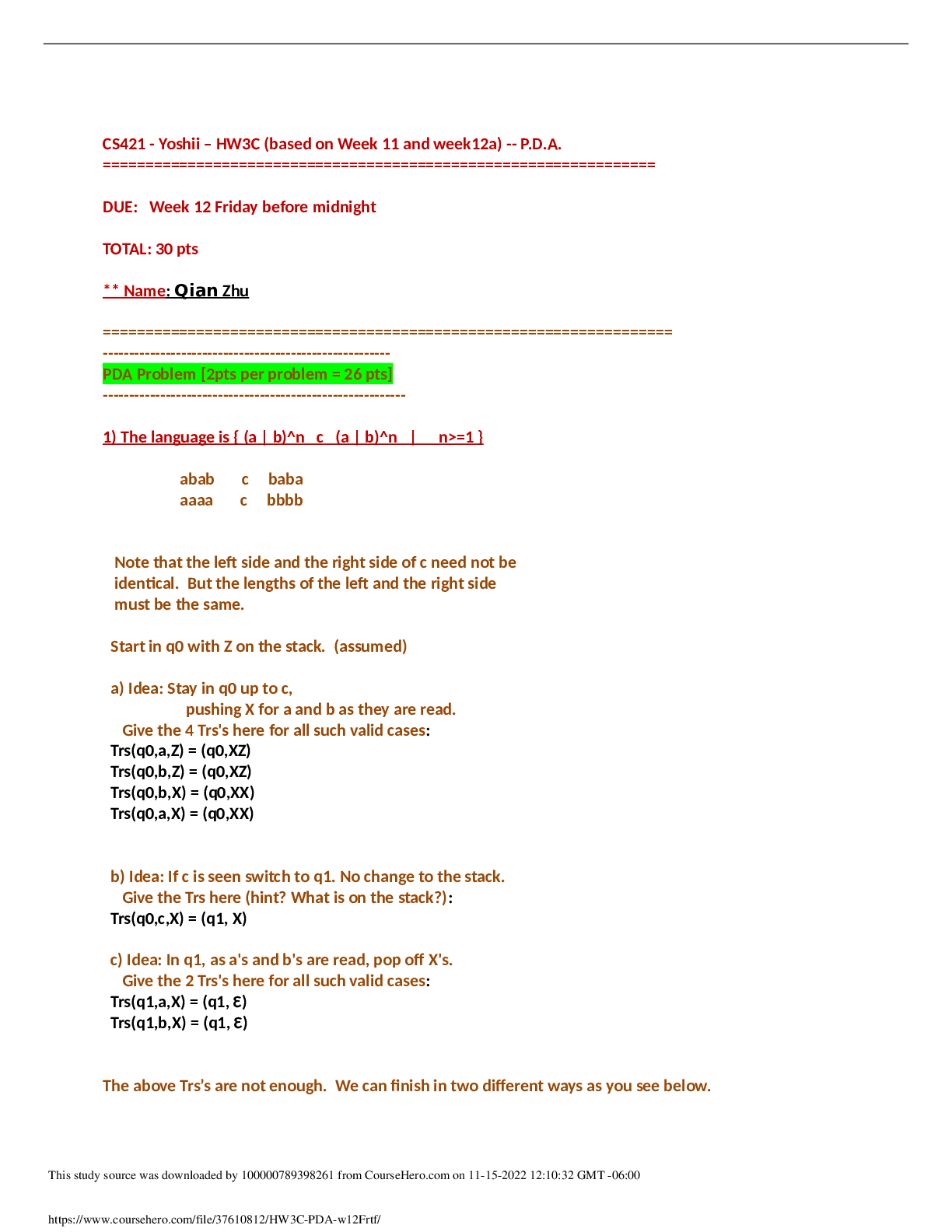
MULTIPLE CHOICE 1. Quality control is designed to: a. ensure the medical reliability of laboratory data. b. check media. c. check reagents. d. monitor incubator and refrigerator temperatures. ... A Checking media, checking reagents, and monitoring incubator and refrigerator temperatures are all included in quality control. Each of these choices represents only a part of quality control, whereas the first choice encompasses all the activities one would do in a quality control program. REF: 94 OBJ: Level 1: Recall 2. Actual laboratory testing is called a(n) _____ activity. a. preanalytic b. analytic c. postanalytic d. research B The analytic activity is the actual running of the laboratory test. Preanalytic activity is what happens before a laboratory test is run. Postanalytic activity is what happens after a laboratory test is run. Research is a term that applies to a series of focused experiments conducted to test a hypothesis. REF: 94 OBJ: Level 1: Recall 3. All of the following activities will directly affect the quality of a laboratory test except: a. preanalytic. b. analytic. c. accreditation. d. postanalytic. C Accreditation is a peer review of the policies and procedures of a laboratory that may indirectly affect the quality of a laboratory test. Laboratory professionals have found that the preanalytic, analytic, and postanalytic activities directly affect the quality of a laboratory test. REF: 94 OBJ: Level 1: Recall 4. In January 2004, The Joint Commission (TJC) implemented performance measurements for organizational systems that are critical to patient safety, quality of care, treatment, and services. This new initiative is called: a. performance improvement. b. quality control. c. quality assurance. d. shared visionsnew pathway. D The new TJC initiative, shared visionsnew pathway, focuses on performance measurement of organizational systems that are critical to patient safety, quality of care, treatment, and services. Quality control consists of processes pertaining to the quality of analytic testing. Performance improvement is a process that consists of setting performance standards, measuring the standards against some criteria, then improving processes that fall short of the standards. Quality assurance is a comprehensive system that takes the preanalytic, analytic, and postanalytic processes into consideration when determining the quality of laboratory data. REF: 94 OBJ: Level 1: Recall 5. All of the following activities are included in a laboratory quality control program except: a. air quality. b. temperatures. c. media. d. antimicrobial susceptibility testing. A The air quality in a laboratory is not routinely tested. A quality control program focuses on procedures, equipment, and policies that affect how well laboratory tests are performed, the quality of submitted specimens, and test results. REF: 94 OBJ: Level 1: Recall 6. The morning technician arrives at the microbiology laboratory and goes around checking daily temperatures. This person will check temperatures daily on all of the following equipment except: a. centrifuges. b. incubators. c. heating blocks. d. refrigerators. A A centrifuge is not typically a temperature-dependent piece of equipment. REF: 94 OBJ: Level 2: Interpretation 7. Thermometers used in the laboratory must be calibrated before they are put into use. This is accomplished by: a. sending to the National Institute of Standards and Technology (NIST) to determine its accuracy. b. checking against an NIST thermometer. c. measuring the grades to make sure they are the standard size and accurate. d. using a colored dye. B A thermometer calibration is conducted by comparing the NIST thermometer reading to the laboratory thermometer’s reading. Any variation is noted on the certificate of calibration. REF: 95 OBJ: Level 1: Recall 8. Preventive maintenance on an instrument includes all the following except: a. disinfecting the surface of the instrument. b. oiling and cleaning the instrument. c. replacing filters. d. recalibrating instruments. A Disinfecting the surface of an instrument is usually done as a daily activity and is generally not considered preventive maintenance. All the other choices are preventive maintenance activities that will keep an instrument in top shape and functioning at the proper level so as to increase its lifetime and keep it producing quality results. REF: 95 OBJ: Level 1: Recall 9. Commercial media must have quality control testing performed by the manufacturer. Because of high failure rates, all of the following media must be retested by the hospital laboratory except: a. Campylobacter media. b. selective media for pathogenic Neisseria. c. trypticase soy agar with 5% sheep blood. d. chocolate. C Trypticase soy agar with 5% sheep blood does not have a high failure rate, so the hospital laboratory need not retest it. REF: 95 OBJ: Level 1: Recall 10. Media that do not need to be retested by the hospital laboratory must still undergo observation for all of the following except: a. moisture. b. sterility. c. breakage. d. organism colony characteristics. D When a laboratory is not required to retest prepared media, it still must observe media for moisture, sterility, breakage, and appearance. Results of media observation must be recorded and must include lot numbers. REF: 96 OBJ: Level 1: Recall 11. When a medium needs to be quality controlled because it was prepared in-house or because it is complex, all the following rules must be followed except: a. only media not specified in Clinical and Laboratory Standards Institute (CLSI) guidelines should be tested. b. the medium should be tested for sterility and pH. c. the organism selected for quality control testing should represent most fastidious organisms for which the medium is designed. d. testing techniques should be different for primary plating media than for biochemical or subculturing media. A All in-house media must be tested for pH, sterility, and positive and negative reacting organisms—that is, if a medium should inhibit a specific organism or group of organisms, it should be tested with those organisms to ensure it “works.” CLSI guidelines give specific guidance as to what type of quality control testing should be performed on media prepared in-house. REF: 98 OBJ: Level 1: Recall 12. Reagents that should undergo quality control in the microbiology laboratory include all the following except: a. optochin. b. immersion oil. c. -lactamase. d. nitrate. B Immersion oil does not need quality control testing. All stains performed in a microbiology laboratory along with optochin, -lactamase, nitrate, bacitracin, catalase, coagulation, gelatin, germ tube, hippurate, Kovac’s, oxidase, PYR, typing sera, Voges-Proskauer, and X&V strips should undergo quality control testing in a microbiology laboratory. REF: 98 OBJ: Level 1: Recall 13. Antimicrobial susceptibility is hard to control because many of the same species of organisms have a varied susceptibility to particular antibiotics. To reduce this variation, Clinical and Laboratory Standards Institute (CLSI) guidelines recommend: a. using in-house organisms isolated from past patients. b. sharing strains between hospital laboratories. c. using specific strains from American Type Culture Collection (ATCC). d. obtaining test strains from Centers for Disease Control and Prevention (CDC). C CLSI recommends specific ATCC strains of control organisms. There is too much variation in organisms isolated from patients to use as quality control organisms. Hospital laboratories trying to share strains would find this a cumbersome process for routine quality control. CDC does not store routine strains of microbes for quality control testing. REF: 99 OBJ: Level 1: Recall 14. In any susceptibility system, variables that can affect the results include: 1. antibiotic potency. 2. inoculum concentration. 3. bactericidal level. 4. pH. 5. anion content. a. 1, 2, and 3 are correct. b. 1, 3, 4, and 5 are correct. c. 1, 2, 3, 4, and 5 are correct. d. 1, 2, and 4 are correct. D The factors affecting results include antibiotic potency, inoculum, concentration, and pH. The bactericidal level of antibiotic and anion content does not affect susceptibility results. Other factors affecting results include agar depth, evaporation, cation content, thymidine content, instrument failure, temperature, moisture, and difficulty in determining endpoints. REF: 99 OBJ: Level 2: Interpretation 15. Susceptibility testing of control organisms is usually conducted daily until precision can be demonstrated with _____ or _____ _____ days of susceptibility testing using CLSI guidelines. a. 20; 30; consecutive b. 15; 20; consecutive c. 30; 40; nonconsecutive d. 5; 10; consistent A Once the 20- or 30-day evaluation has been accomplished, quality control organisms may be tested weekly instead of daily. All results should be kept as long as the antimicrobial agent is used or at least 2 years after discontinuing the agent. REF: 100 OBJ: Level 1: Recall 16. How can personnel competency be determined? a. Asking an employee to explain how to do a test b. Proficiency testing c. Asking another employee to comment on someone’s competency d. Assuming competency from one’s credentials B Proficiency testing consists of carefully designing samples and giving them to technicians as unknowns for the purpose of identifying them. This will show how competent a technician is at performing job tasks. Asking an employee how to do a test is not the same as having someone perform the test. Doing a test involves motor skills and coordination that cannot be measured by asking a person about how to do a test. Two things that are never done to evaluate a person’s competency are asking another employee to comment on someone’s competency and assuming one is competent from his or her credentials. REF: 100 OBJ: Level 1: Recall 17. All of the following are provisions of CLIA 88 except: a. verify employee competency upon employment. b. determine an employee’s competency. c. reverify the employee’s competency monthly. d. reverify the employee’s competency annually. C To maintain quality in the laboratory, CLIA 88 mandated that an employee’s competency must be verified upon employment, an employer must determine an employee’s competency, and the employee’s competency must be reverified annually. It did not mandate reverifying an employee’s competency monthly because this would be an impossible task—the time frame is too short. REF: 100 OBJ: Level 1: Recall 18. Quality control stock cultures are available from all of the following except: a. American Type Culture Collection (ATCC). b. commercial sources. c. proficiency testing isolates. d. Centers for Disease Control and Prevention (CDC). D CDC is a clearinghouse for knowledge concerning infectious diseases, but CDC does not provide stock cultures to hospital laboratories. REF: 100 OBJ: Level 1: Recall 19. Popular media choices for maintaining stock cultures include: a. TSA deeps. b. cornmeal. c. Sabouraud dextrose. d. CTA with glucose. A TSA deeps is the only choice that is sugar-free. For best results, a stock culture should be grown in a large volume of broth, then divided among enough small freezer vials to last a year. Media selection for freezing is at the discretion of the individual laboratory, but it should not contain sugars. If organisms use sugars while being maintained, acid by-products could kill the organism over time. CTA, Sabouraud dextrose, and cornmeal all have sugar. REF: 100 OBJ: Level 1: Recall 20. Which of the following is an example of a mission statement for an institution? a. “The AFMS Flagship—Comprehensive Healthcare…On Time…On Target” b. “Working Together for a Healthy America” c. “Your Total eLearning Solution” d. “Will Answer Questions Correctly 95% of the Time” B An organization’s vision and mission statements must be clearly emphasized so that all employees become involved and understand that each one of them has a role in the mission, and that they must strive to make performance improvement part of their everyday life to achieve the organization’s mission. The first and third choices are vision statements, whereas the last choice is an objective. REF: 102 OBJ: Level 2: Interpretation 21. All the following are components of The Joint Commission recommendations for establishing performance monitors except: a. plan. b. design. c. do. d. assess. C The components of the recommendations are plan, design, measure, and assess. REF: 102 OBJ: Level 1: Recall 22. Customers of a laboratory include all the following except: a. patients. b. insurers. c. doctors. d. accrediting organizations. D Anyone who looks to the laboratory for service is a customer. Accrediting organizations are not expecting services from a laboratory, so they cannot be considered a customer. Doctors, nurses, insurers, and patients are all customers. REF: 103 OBJ: Level 1: Recall 23. An unlabeled specimen is sent from the intensive care unit to the laboratory. When the specimen arrives in the laboratory, the laboratory manager decides that corrective action needs to occur. What can be constructed to evaluate and correct the problem? a. Find fault with the nurse causing the problem. b. Provide training for the nurse responsible for the problem. c. Empower a facilitator. d. Make a cross-functional team. D When patient outcome is less than desirable, the process must be evaluated and corrected. The focus is on the process, not the individual. The primary rule to follow is to refrain from finger pointing or fault finding. Preanalytic and postanalytic activities usually take place outside the laboratory and require a cross-functional team to evaluate and correct the problem. REF: 103 OBJ: Level 3: Synthesis 24. A broken process is fixed when: a. the problem is prevented from happening again. b. everyone is happy with the process. c. the process becomes seamless. d. the hospital administrator determines that the cross-functional team is no longer needed. A A process is fixed only when the problem is prevented from happening again. It is not fixed just because the reason something went wrong is explained. REF: 103 OBJ: Level 1: Recall 25. What is benchmarking? a. Identifying a problem to be fixed b. Seeking an industry’s or profession’s best practices to imitate and improve c. Explaining why a problem occurred d. Asking the CEO’s opinion and going with his or her idea B Benchmarking is seeking an industry’s or profession’s best practice to imitate and improve. Benchmarking was initially practiced in business and industry, but it has now become an important part of the hospital quality management program. REF: 103 OBJ: Level 1: Recall 26. When performing tests in the microbiology laboratory, the detection limit of a test refers to the _____ of that test. a. analytic specificity b. analytic sensitivity c. clinical sensitivity d. clinical specificity B The analytic sensitivity of a test refers to its ability to detect a particular analyte or a small change in its concentration. Analytic sensitivity is usually defined as the 0.95 confidence level (+/– 2 SD) and may be referred to as the detection limit. Analytic specificity is a test’s ability not to react with substances other than the one of interest. Clinical sensitivity is the proportion of specimens that test positive from people who are known to have the disease. Clinical specificity is the population of specimens that test negative from those known to be disease free. REF: 105 OBJ: Level 1: Recall 27. A technician is performing a chemistry test on a control sample. The technician gets the following values for the control: 4.3, 4.3, 4.3, 4.2, 4.4, 4.3, 4.2, and 4.4. The actual value is 4.3. These results are said to be: a. systematically inaccurate. b. sensitive. c. accurate. d. bias. C The degree of conformity of a measurement to a standard or true value is accuracy. Bias is the mean difference of test results from an accepted reference method caused by systematic errors. REF: 105 OBJ: Level 3: Synthesis 28. A test is performed on a group of 10 patients. Six patients have a disease, and four are free of the disease. If six patients (all known to have the disease) of this group test positive for the disease, we say that the clinical sensitivity of this test is: a. 60%. b. 40%. c. 50%. d. 100%. D Clinical sensitivity is the proportion of positive test results obtained when a test is applied to patients known to have the disease. Thus it is the frequency of positive test results in patients with the disease. If six patients in our group are known to have the disease and all six test positive, then the clinical sensitivity of the test is 100%. REF: 105 OBJ: Level 3: Synthesis 29. A group of 10 patients are to be tested: six are known to have a disease and four are disease free. If the four disease-free patients tested negative for this disease, what would the clinical specificity of this test be? a. 100% b. 60% c. 40% d. 50% A Clinical specificity is the proportion of negative results obtained when a test is applied to patients known to be free of disease. Thus it is the frequency of negative test results in patients without the disease. So if there are four patients in this group and they all test negative with this test, then the clinical specificity is 100%—all negative individuals tested negative with this test. REF: 106 OBJ: Level 3: Synthesis 30. Incidence is: a. the frequency of a disease at a designated single point in time in the population being tested. b. the number of new cases of disease over a period of time. c. proportion of negative test results in a population known to be free of disease. d. positive results in patients with the disease. B Incidence is defined as the number of new cases of disease over a period of time. Prevalence is the frequency of a disease at a designated single point in time in the population being tested. Clinical specificity is the proportion of negative test results in a population known to be free of the disease. Clinical sensitivity is the positive results in patients with the disease. REF: 106 OBJ: Level 1: Recall 31. A test can have both a positive and negative predictive value. All the following elements are needed to compute the positive and negative predictive value of a test except: a. sensitivity of a test. b. specificity of a test. c. type of disease being tested. d. prevalence of the disease being tested. C The predictive value of a test is the probability that a positive result (positive predictive value) accurately indicates the presence of an analyte or a specific disease. The formula follows. where PPV = positive predictive value P = prevalence of disease being tested Se = sensitivity of test Sp = specificity of test REF: 106 OBJ: Level 1: Recall 32. Assume that a certain test for group A Streptococcus has reported sensitivity and specificity of 90% and 98%, respectively, and the estimated prevalence for group A Streptococcus infection in acute pharyngitis is 5%. What is the positive predictive value of this test? a. 99.5% b. 30% c. 90% d. 70.3% D where PPV = positive predictive value P = prevalence of disease being tested Se = sensitivity of test Sp = specificity of test REF: 107 OBJ: Level 3: Synthesis 33. Usually, negative test results are more reliable in predicting the: a. absence of disease. b. presence of disease. c. sensitivity of a test. d. specificity of a test. A Usually, with tests, the negative predictive value (NPV) is higher than the positive predictive value (PPV). This shows that tests can more accurately predict the absence of disease. The sensitivity and specificity of a test do not predict the presence or absence of a disease. REF: 107 OBJ: Level 1: Recall 34. The efficiency of a test indicates: a. the percentage of patients who test positive for the disease. b. the percentage of patients who are correctly identified as having or not having the disease. c. the percentage of patients who test negative for the disease. d. the number of patients tested for a disease. B Test efficiency = TP = number of patients with true positive TN = number of patients with true negative FP = number of patients with false positive FN = number of patients with false negative REF: 107 OBJ: Level 1: Recall 35. Screening is: a. used for only particular test analytes. b. not practical in a hospital laboratory. c. used for testing large populations of patients. d. when a high incidence of a disease is found in a population. C Screening is used for testing large populations of patients. Generally, screening tests have high clinical sensitivity and negative predictive value. Positive results with such tests generally require confirmation with a more specific test. REF: 108 OBJ: Level 1: Recall 36. Confirmation is used after: a. careful consideration by the doctor to do the test. b. a test that has a low predictive value. c. a test that has a low specificity. d. obtaining a positive screening result. D Confirmation is the process of repeating a screening test using a test that is more sensitive and specific to ensure accuracy for the initial screening result. Confirmatory tests help seek out false-positive screening tests. REF: 109 OBJ: Level 1: Recall 37. When choosing a test to perform in your laboratory, an in-house verification is always performed. Verification of a test: a. serves to establish that the performance parameters of the test are satisfactory. b. lets technicians actually perform the test. c. lets physicians perform the test. d. allows laboratory management to view positive results. A A test must be verified by a laboratory to ensure that it will work as described on the package. A test must perform up to specifications in a laboratory or it is not worth using. Although having technicians perform the test in-house shortens the turnaround time for results, the test is evaluated for its performance. Physicians are not allowed to perform tests in a clinical laboratory. Laboratory management does not need to see positive test results. Laboratory management will evaluate the test on its in-house performance. REF: 109 OBJ: Level 1: Recall 38. Test validation is the ongoing process of providing information that a test is performing correctly. The components of validation include all the following except: a. quality control. b. specimen requirements. c. proficiency testing. d. instrument calibration. B Specimen requirements do not assess the functioning of a test. Quality control, proficiency testing, and instrument calibration evaluate test functioning on a continual basis. REF: 109 OBJ: Level 1: Recall 39. All of the following provide guidelines to ensure the continual correct performance of tests except: a. accrediting agencies. b. regulatory agencies. c. Centers for Disease Control and Prevention (CDC). d. manufacturers. C CDC does not publish recommendations in regard to continual test validation. CDC is a clearinghouse for infectious disease knowledge. REF: 109 OBJ: Level 1: Recall [Show More]
Last updated: 2 years ago
Preview 1 out of 12 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 23, 2020
Number of pages
12
Written in
Additional information
This document has been written for:
Uploaded
Jan 23, 2020
Downloads
0
Views
78




.png)
.png)
.png)
.png)
.png)
.png)
.png)

