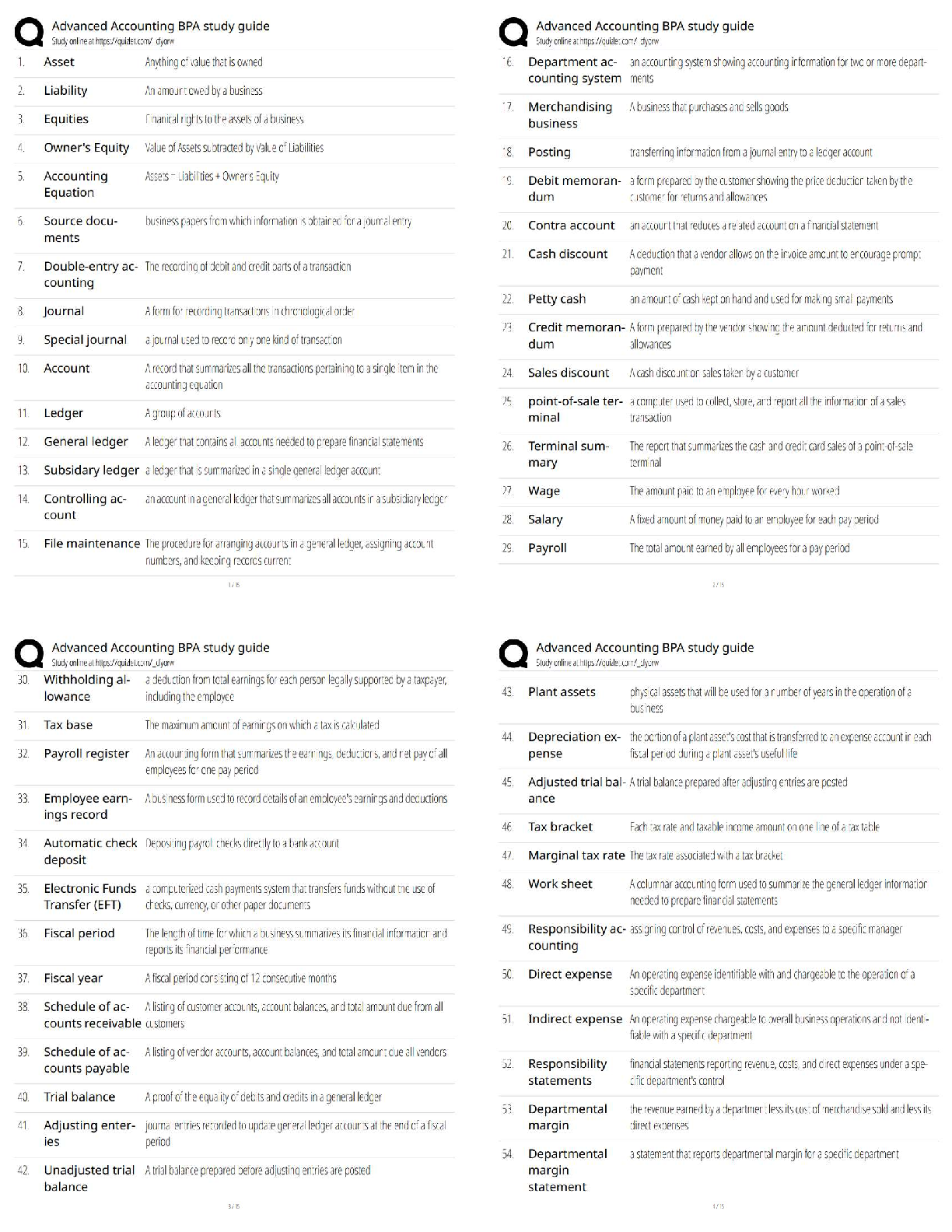
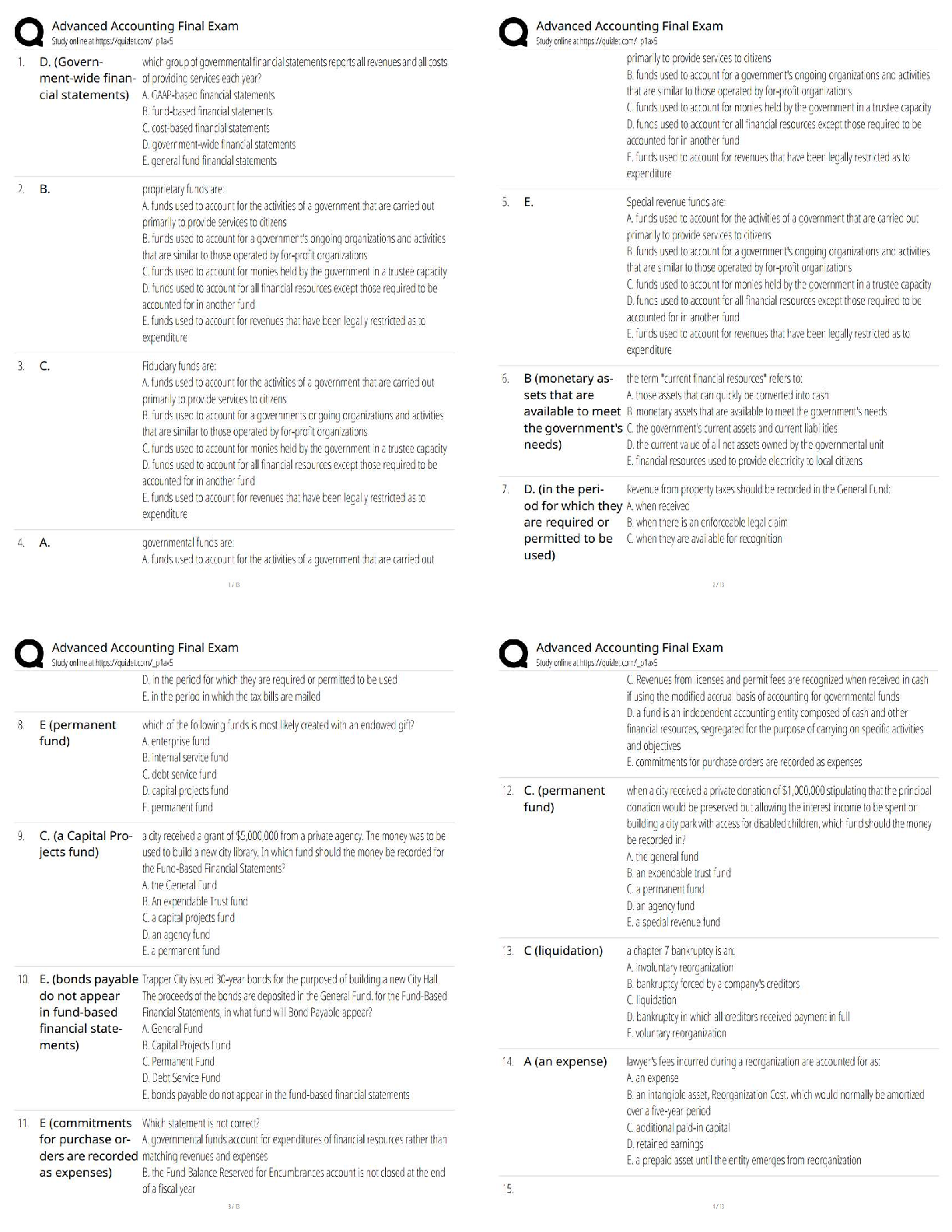
Multiple Choice
1. What are the fundamental building blocks of all matter?
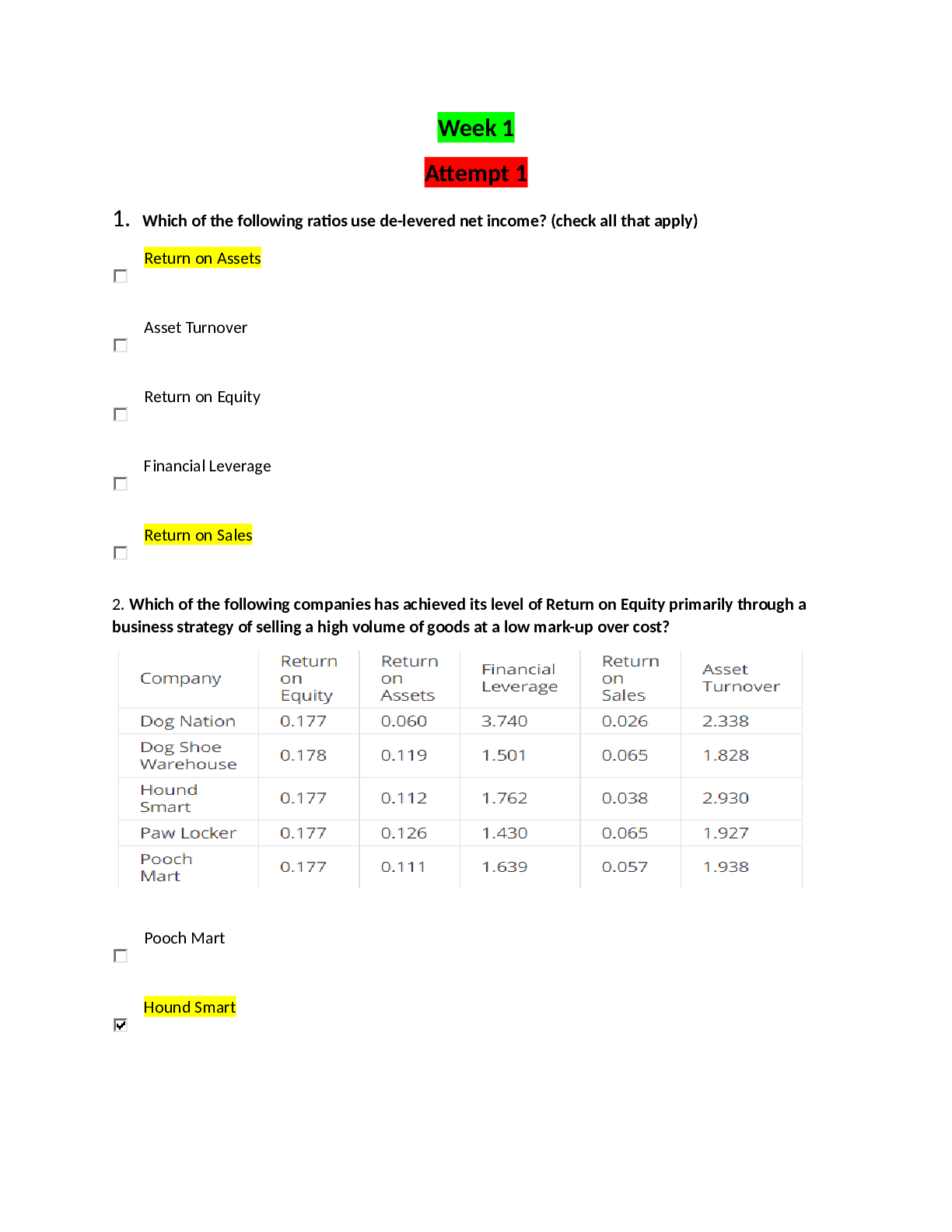
a. atoms
b. compounds
c. ions
d. molecules
e. electrons
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic bu
...
Multiple Choice
1. What are the fundamental building blocks of all matter?
a. atoms
b. compounds
c. ions
d. molecules
e. electrons
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1 - Describe a few properties of atoms and elements.
KEYWORDS: Bloom's: Remember
NOTES: Modified
2. Negatively charged subatomic particles are known as:
a. neutrons
b. protons
c. electrons
d. elements
e. atoms
c
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.1 - What is an atom composed of?
KEYWORDS: Bloom's: Remember
NOTES: Modified
Selecting the Exception
3. Four of the five answers are elements. Select the exception:
a. water
b. oxygen
c. carbon
d. chlorine
e. hydrogen
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1 - Describe a few properties of atoms and elements.
KEYWORDS: Bloom's: Understand
NOTES: Modified
4. Four of the five answers below are correct statements about electrons. Select the exception.
a. Electrons closest to the nucleus are at the lowest energy level.
b. No more than two electrons can occupy a single orbital.
c. Electrons cannot move out of their assigned orbital space.
d. The innermost orbital holds two electrons.
e. At the second energy level, there are four possible orbitals with a total of eight electrons.
c
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.1 - Explain how electrons are organized in orbitals.
KEYWORDS: Bloom's: Remember
NOTES: Modified
5. Four of the five answers listed below possess electrons in the third energy level. Select the exception.
a. sodium (11)
b. magnesium (12)
c. chlorine (17)
d. neon (10)
e. argon (18)
d
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Apply
OTHER: Selecting the Exception
6. Four of the five answers listed below are characteristics of water. Select the exception.
a. Water stabilizes temperature.
b. Water is an excellent solvent.
c. Water has cohesion and surface tension.
d. Water produces salts.
e. Water is less dense when solid.
d
POINTS: 1
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.3 - Why is water a good solvent?
KEYWORDS: Bloom's: Remember
OTHER: Selecting the Exception
7. Which of the following is a positive subatomic particle?
a. neutron only
b. proton only
c. electron only
d. neutron and proton
e. proton and electron
b
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.1 - What is an atom composed of?
KEYWORDS: Bloom's: Remember
8. Which of the following is a neutral subatomic particle?
a. neutron
b. proton
c. electron
d. neutron and proton
e. electron and neutron
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.1 - What is an atom composed of?
KEYWORDS: Bloom's: Remember
9. The atomic number is determined by the number of:
a. neutrons and protons
b. neutrons and electrons
c. protons and electrons
d. protons only
e. neutrons only
d
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.3 - Describe how atoms are arranged in the periodic table.
KEYWORDS: Bloom's: Remember
10. All atoms of an element have the same number of:
a. ions
b. protons only
c. neutrons only
d. electrons.
e. protons and neutrons
b
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.2 - What is an element?
KEYWORDS: Bloom's: Remember
11. The nucleus of an atom contains:
a. neutrons and protons
b. neutrons and electrons
c. protons and electrons
d. protons only
e. neutrons only
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.1 - What is an atom composed of?
KEYWORDS: Bloom's: Remember
12. The mass number of an atom is determined by the weight of:
a. neutrons and protons
b. neutrons and electrons
c. protons and electrons
d. protons only
e. neutrons only
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.3 - Describe how atoms are arranged in the periodic table.
KEYWORDS: Bloom's: Remember
NOTES: Modified
13. An atom of sodium has an atomic number of 11 and a mass of 23. How many neutrons does it have?
a. 11
b. 12
c. 23
d. 34
e. 35
b
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.3 - Describe how atoms are arranged in the periodic table.
KEYWORDS: Bloom's: Apply
14. Carbon has several isotypes including 12C and 14C. These isotopes differ in the number of:
a. electrons only
b. neutrons only
c. protons only
d. electrons and protons
e. protons and neutrons
b
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.4 - What are isotopes?
KEYWORDS: Bloom's: Remember
NOTES: Modified
15. When 14C goes through radioactive decay, one of its neutrons splits into a proton and an electron. What does 14C become?
a. 13C6
b. 14N7
c. 13N7
d. 13B5
e. 15C6
b
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.4 - What are isotopes?
KEYWORDS: Bloom's: Apply
NOTES: Modified
16. In the shell model, the second shell can hold up to _____ electrons.
a. one
b. two
c. four
d. six
e. eight
e
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Remember
17. For an atom to be neutral, it must have the same number of:
a. electrons and neutrons
b. electrons and protons
c. neutrons and protons
d. neutrons only
e. electrons, neutrons, and protons
b
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2 - Describe the arrangement of electrons in atoms.
KEYWORDS: Bloom's: Apply
18. Which subatomic particles are arranged in various energy levels or orbitals?
a. electrons only
b. protons only
c. neutrons only
d. electrons and protons
e. protons and neutrons
a
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.1 - Explain how electrons are organized in orbitals.
KEYWORDS: Bloom's: Remember
NOTES: Modified
19. Water is an example of a(n):
a. atom
b. ion
c. compound
d. mixture
e. element
c
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3 - Describe the different types of chemical bonds.
KEYWORDS: Bloom's: Remember
20. A molecule is:
a. a combination of two or more atoms
b. less stable than its constituent atoms separated
c. electrically charged
d. a carrier of one or more extra neutrons
e. another term for an atom
a
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3 - Describe the different types of chemical bonds.
KEYWORDS: Bloom's: Remember
21. Magnesium has 12 protons. How many electrons are in its third energy level?
a. two
b. four
c. six
d. eight
e. ten
a
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Apply
22. Magnesium has 12 protons. How many electrons are in its first energy level?
a. 2
b. 4
c. 6
d. 8
e. 10
a
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Apply
23. Magnesium has 12 protons. How many electrons are in its second energy level?
a. two
b. four
c. six
d. eight
e. ten
d
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Apply
24. Of the following, an atom with the atomic number ____ would be the least reactive.
a. 1
b. 3
c. 17
d. 18
e. 21
d
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.4 - Why are atoms with vacancies said to be chemically reactive?
KEYWORDS: Bloom's: Understand
25. What is formed when an atom loses or gains an electron?
a. a new element
b. ion
c. molecule
d. bond
e. isotope
b
POINTS: 1
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.6 - How do atoms become ions?
KEYWORDS: Bloom's: Remember
26. The bond in table salt (NaCl) is:
a. polar
b. ionic
c. covalent
d. double
e. nonpolar
b
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3.2 - With the help of suitable examples, distinguish between ionic and covalent bonds.
KEYWORDS: Bloom's: Remember
27. What type of bond is formed whenever atoms share a pair of electrons?
a. covalent
b. hydrogen
c. ionic
d. double
e. peptide
a
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3.2 - With the help of suitable examples, distinguish between ionic and covalent bonds.
KEYWORDS: Bloom's: Understand
28. What type of bond is(are) individually weakest?
a. hydrogen
b. ionic
c. covalent
d. Hydrogen and covalent are equally weak.
e. Ionic and covalent are both weaker than hydrogen.
a
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3.3 - Define polarity and explain why ionic bonds are extremely polar.
KEYWORDS: Bloom's: Remember
NOTES: Modified
29. A hydrogen bond is:
a. a sharing of a pair of electrons between a hydrogen and an oxygen nucleus
b. a sharing of a pair of electrons between a hydrogen nucleus and either an oxygen or a nitrogen nucleus
c. a weak attraction between a covalently bonded hydrogen atom and another atom taking part in a separate polar covalent bond
d. the loss of an electron by hydrogen to a highly electronegative atom
e. a strong chemical bond between two ions
c
POINTS: 1
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.2 - Describe the nature of a hydrogen bond.
KEYWORDS: Bloom's: Remember
NOTES: Modified
30. Which of the following is(are) classified as true chemical bonds?
a. hydrogen only
b. ionic only
c. covalent only
d. both ionic and covalent
e. hydrogen, ionic and covalent
d
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
Section 2.4 What are life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.3.2 - With the help of suitable examples, distinguish between ionic and covalent bonds.
KEYWORDS: Bloom's: Understand
NOTES: Modified
31. How do hydrophobic molecules react with water?
a. They are attracted to water.
b. They are absorbed by water.
c. They are repelled by water.
d. They are mixed with water.
e. They are polarized by water.
c
POINTS: 1
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.5 - Distinguish between hydrophilic and hydrophobic substances.
KEYWORDS: Bloom's Remember
32. Why does ice float on water?
a. Ice is hydrophobic and repels water.
b. Water molecules have less mass the colder they get.
c. Water molecules are spaced farther apart in ice than in liquid water.
d. Vibrating electrons in liquid water push ice to the surface.
e. Hydrogen bonds are weaker in ice.
c
POINTS: 1
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.8 - Explain how the movement of water molecules varies with temperature.
KEYWORDS: Bloom's Understand
NOTES: Modified
33. A salt will dissolve in water to form:
a. acids
b. gases
c. ions
d. bases
e. polar solvents
c
POINTS: 1
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.4 - Describe the mechanism by which different kinds of substances dissolve in water.
KEYWORDS: Bloom's: Remember
34. Of the following, pH ____ is the most acidic.
a. 1
b. 3
c. 6
d. 7
e. 8
a
POINTS: 1
REFERENCES: Section 2.5 Why are hydrogen ions important in biological systems?
LEARNING OBJECTIVES: BCA.SES.2.5.3 - Differentiate between strong acids and weak acids with examples.
KEYWORDS: Bloom's: Remember
35. Cellular pH is kept near a value of seven, due to the action of:
a. salts
b. buffers
c. acids
d. bases
e. water
b
POINTS: 1
REFERENCES: Section 2.5 Why are hydrogen ions important in biological systems?
LEARNING OBJECTIVES: BCA.SES.2.5.4 - Explain how buffers maintain the pH of solutions.
KEYWORDS: Bloom's: Remember
36. Substances that are _____ give up hydrogen ions when they dissolve in water.
a. basic
b. acidic
c. neutral
d. hydrophobic
e. buffered
b
POINTS: 1
REFERENCES: Section 2.5 Why are hydrogen ions important in biological systems?
LEARNING OBJECTIVES: BCA.SES.2.5.2 - Differentiate between acids and bases.
KEYWORDS: Bloom's: Remember
37. In the periodic table, symbols for the elements are arranged according to _____.
a. size
b. charge
c. mass number
d. atomic number
e. electronegativity gradient
d
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.3 - Describe how atoms are arranged in the periodic table.
NOTES: Modified
38. The measure of an atom’s ability to pull electrons away from another atom is called _____.
a. electronegativity
b. polarity
c. charge
d. concentration
e. atomic number
a
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3.4 - What is electronegativity and what does it depend on?
NOTES: New
39. When dissolved in water, a(n) _____ donates H+; a(n) _____accepts H+.
a. acid; base
b. base; acid
c. buffer; solute
d. base; buffer
e. solvent; solute
a
POINTS: 1
REFERENCES: Section 2.5 Why are hydrogen ions important in biological systems?
LEARNING OBJECTIVES: BCA.SES.2.5.6 - Explain why it is important to maintain the pH of biological systems within a consistent range.
NOTES: Modified
Figure 2.1
40. In the accompanying figure, the atomic number refers to the:
a. number of neutrons and protons in the atomic nucleus
b. number of neutrons in the orbital
c. number of electrons in the atomic nucleus
d. number of protons in the atomic nucleus
e. total number of electrons, neutrons, and proteins in the atom
d
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.2 - What is an element?
NOTES: New
41. In the accompanying figure, how many neutrons does the carbon element have?
a. one
b. three
c. six
d. nine
e. twelve
c
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.2 - What is an element?
KEYWORDS: Bloom's Apply
NOTES: New
42. In the accompanying figure, how many protons does the carbon element have?
a. one
b. thee
c. six
d. nine
e. twelve
c
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.2 - What is an element?
KEYWORDS: Bloom's: Apply
NOTES: New
43. What is the process in which a nucleus of an atom breaks up, emitting subatomic particles and/or energy?
a. radioactive decay
b. radioactive isotyping
c. unstable atomic nucleus
d. electron release
e. free radical release
a
POINTS: 1
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
LEARNING OBJECTIVES: BCA.SES.2.1.4 - What are isotopes?
KEYWORDS: Bloom's: Remember
NOTES: New
44. Molecular oxygen is composed of two oxygen atoms that share four electrons. How many covalent bonds exist between the two oxygen atoms?
a. none
b. one
c. two
d. four
e. eight
c
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.3.5 - Describe how covalent bonds between atoms are represented.
KEYWORDS: Bloom's: Understand
NOTES: New
45. Sodium (Na) atoms often lose the single electron that is in their outermost shell. How does losing the electron affect sodium's charge?
a. It is neutral.
b. It has a positive charge.
c. It has a negative charge.
d. It increases its electronegativity.
e. The charge is not affected by the electron loss.
b
POINTS: 1
REFERENCES: Section 2.2 How do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.3.3 - Define polarity and explain why ionic bonds are extremely polar.
KEYWORDS: Bloom's: Understand
NOTES: New
46. Water molecules consist of one oxygen atom and two hydrogen atoms. In these covalent bonds, the electrons are pulled towards the oxygen side of the covalent bond, making one side of the molecule slightly negative and the other side of the molecule slightly positive. What is this characteristic of water called?
a. polarity
b. ionic
c. hydrophilic
d. hydrophobic
e. cohesion
a
POINTS: 1
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.3.3 - Define polarity and explain why ionic bonds are extremely polar.
BCA.SES.2.4.1 - Draw the hydrogen bond between two water molecules.
KEYWORDS: Bloom's: Understand
NOTES: New
47. Hydrogen bonds in water collectively exert a continuous pull on its individual molecules. Therefore, water molecules resist separating from each other. This property is called:
a. ionic bonding
b. solvency
c. polarity
d. hydrophilic tension
e. cohesion
e
POINTS: 1
REFERENCES: Section 2.4 What are the life sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4.2 - Describe the nature of a hydrogen bond.
KEYWORDS: Bloom's: Understand
NOTES: New
48. What is the pH of a solution when the number of H+ ions equals the number of OH– ions in the liquid?
a. 0-2
b. 3-6
c. 7
d. 8-10
e. 11-14
c
POINTS: 1
REFERENCES: Section 2.5 Why are hydrogen ions important in biological systems?
LEARNING OBJECTIVES: BCA.SES.2.5.1 - What is pH?
KEYWORDS: Bloom's: Apply
NOTES: New
Matching
The various energy levels in an atom of chlorine (atomic number 17) have different numbers of electrons. Use the following numbers to answer the question(s).
a. 1
b. 2
c. 3
d. 7
e. 8
REFERENCES: Section 2.2 Why do atoms interact?
LEARNING OBJECTIVES: BCA.SES.2.2.2 - Explain the shell model of electrons with an example.
KEYWORDS: Bloom's: Apply
OTHER: Classification Questions
49. Number of electrons in the first energy level
b
POINTS: 1
50. Number of electrons in the second energy level
e
POINTS: 1
51. Number of electrons in the third energy level
d
POINTS: 1
Match the terms with their most suitable description.
a. protons > electrons
b. number of protons in nucleus
c. polar; easily dissolves in water
d. ion
e. protons < electrons
f. protons = electrons
g. measure of molecular motion
h. number of protons and neutrons in atomic nucleus
REFERENCES: Section 2.1 What are the basic building blocks of all matter?
Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.1 - Describe a few properties of atoms and elements.
BCA.SES.2.3 - Describe the different types of chemical bonds.
52. hydrophilic
c
POINTS: 1
53. atomic number
b
POINTS: 1
54. charged atom
d
POINTS: 1
55. mass number
h
POINTS: 1
56. temperature
g
POINTS: 1
57. uncharged
f
POINTS: 1
58. negative charge
e
POINTS: 1
59. positive charge
a
POINTS: 1
Match the following terms with the best description.
a. compound
b. polarity
c. molecule
d. electronegativity
e. ion
REFERENCES: Section 2.2 Why do atoms interact?
Section 2.3 How do atoms interact in chemical bonds?
LEARNING OBJECTIVES: BCA.SES.2.2 - Describe the arrangement of electrons in atoms.
BCA.SES.2.3 - Describe the different types of chemical bonds.
KEYWORDS: Bloom's Understand
NOTES: New
60. separation of charge into positive and negative regions of a molecule
b
POINTS: 1
61. an atom's ability to pull electrons away from another atom
d
POINTS: 1
62. a type of molecule that has atoms of two or more elements
a
POINTS: 1
63. a charged atom
e
POINTS: 1
64. atoms linked by a chemical bond
c
POINTS: 1
Match the chemical bond descriptions with the type of bond listed below: (each answer may be used more than once).
a. ionic bond
b. covalent bond
c. hydrogen bond
d. polar covalent bond
REFERENCES: Section 2.3 How do atoms interact in chemical bonds?
Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.3 - Describe the different types of chemical bonds.
BCA.SES.2.4 - Describe the properties that liquid water acquires due to hydrogen bonding.
KEYWORDS: Bloom's: Remember
NOTES: New
65. forms between atoms with little to no difference in electronegativity
b
POINTS: 1
66. sharing of electrons between two atoms
b
POINTS: 1
67. links ions of opposite charge
a
POINTS: 1
68. stabilizes the characteristic structures of biological molecules such as DNA and proteins
c
POINTS: 1
69. ions retain their respective charges in this bond
a
POINTS: 1
70. attraction between a covalently bonded hydrogen atom and another atom taking part in a separate covalent bond
c
POINTS: 1
71. where atoms share electrons unequally
d
POINTS: 1
72. single, double, or triple bonds can exist between two atoms
b
POINTS: 1
Imagine that you are pouring some table salt (NaCl) into a glass of water and stirring it until the salt has dissolved. Match the following terms with the best description.
a. solvent
b. solute
c. solution
REFERENCES: Section 2.4 What are the life-sustaining properties of water?
LEARNING OBJECTIVES: BCA.SES.2.4 - Describe the properties that liquid water acquires due to hydrogen bonding.
KEYWORDS: Bloom's: Apply
NOTES: New
73. The glass of water with the salt dissolved in it
c
POINTS: 1
74. The water
a
POINTS: 1
75. The salt
b
POINTS: 1
Subjective Short Answer
76. Why does carbon dioxide dissolved in water act as a buffer?
77. Explain why atoms such as helium, neon, and argon do not react with other atoms.
78. How do ectothermic (cold-blooded) animals make use of water's temperature-stabilizing effects?
79. How can radioisotopes be used as tracers to study biological processes?
80. Overall, a water molecule has no charge but it is a polar molecule. What makes water polar and how does it's polarity contribute to hydrogen bonding?
81. What makes water an excellent solvent?
82. What is the difference between strong and weak acids?
Essay
83. Alchemists were medieval scholars and philosophers who were the forerunners of modern-day chemists. Many tried repeatedly to transform lead (atomic number 82) into gold (atomic number 79). Explain why they never did succeed in that endeavor.
84. Draw a shell model of an uncharged nitrogen atom (nitrogen has 7 protons).
85. Polonium is a rare element with 33 radioisotopes. The most common one, 210Po, has 82 protons and 128 neutrons.When 210Po decays, it emits an alpha particle, which is a helium nucleus (2 protons and 2 neutrons). 210Po decay is tricky to detect because alpha particles do not carry very much energy compared to other forms of radiation. They can be stopped by, for example, a sheet of paper or a few inches of air. That is one reason that authorities failed to discover toxic amounts of 210Po in the body of former KGB agent Alexander Litvinenko until after he died suddenly and mysteriously in 2006. What element does an atom of 210Po change into after it emits an alpha particle?
[Show More]








.png)
.png)
.png)
.png)