Boston College
CHEM CH111
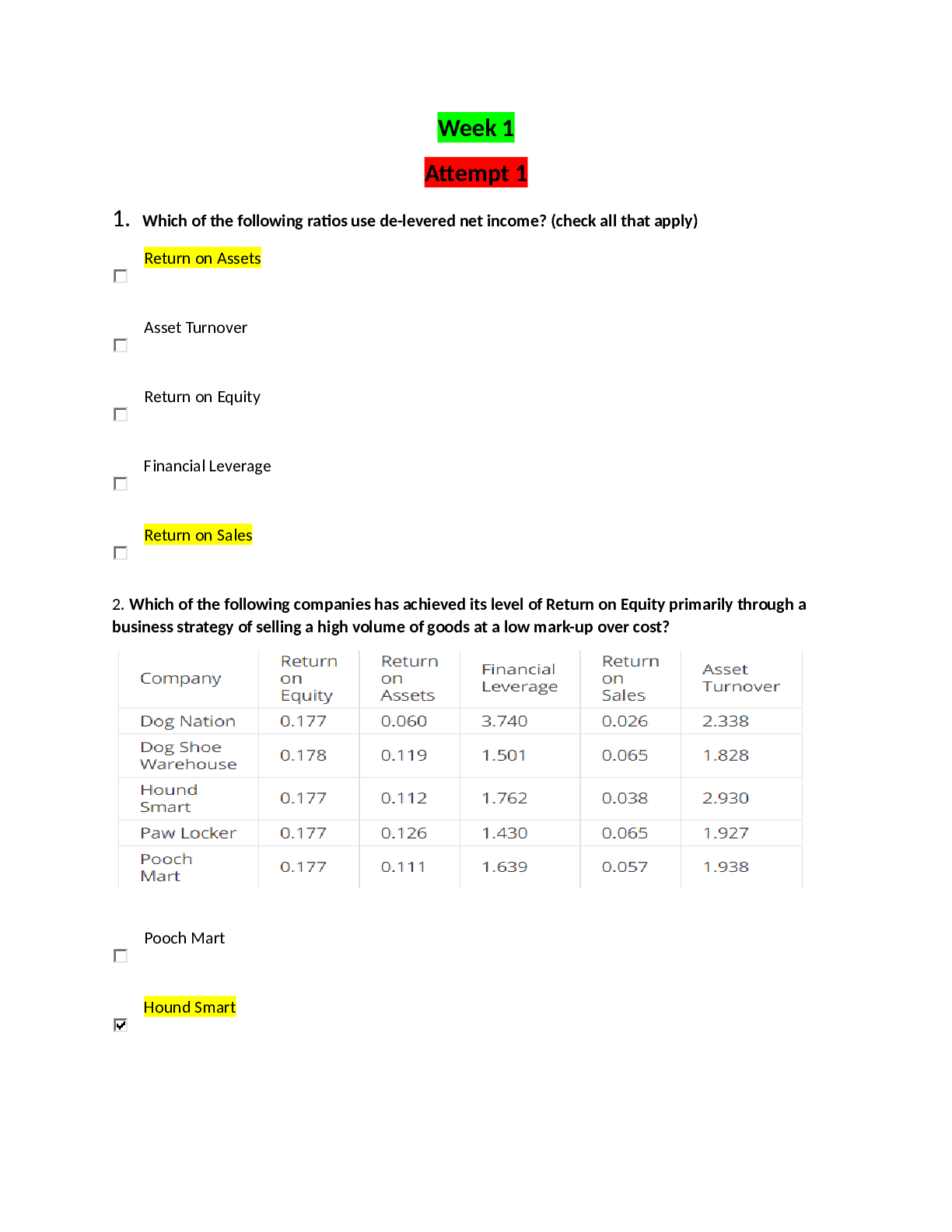
Experiment 9 post Lab
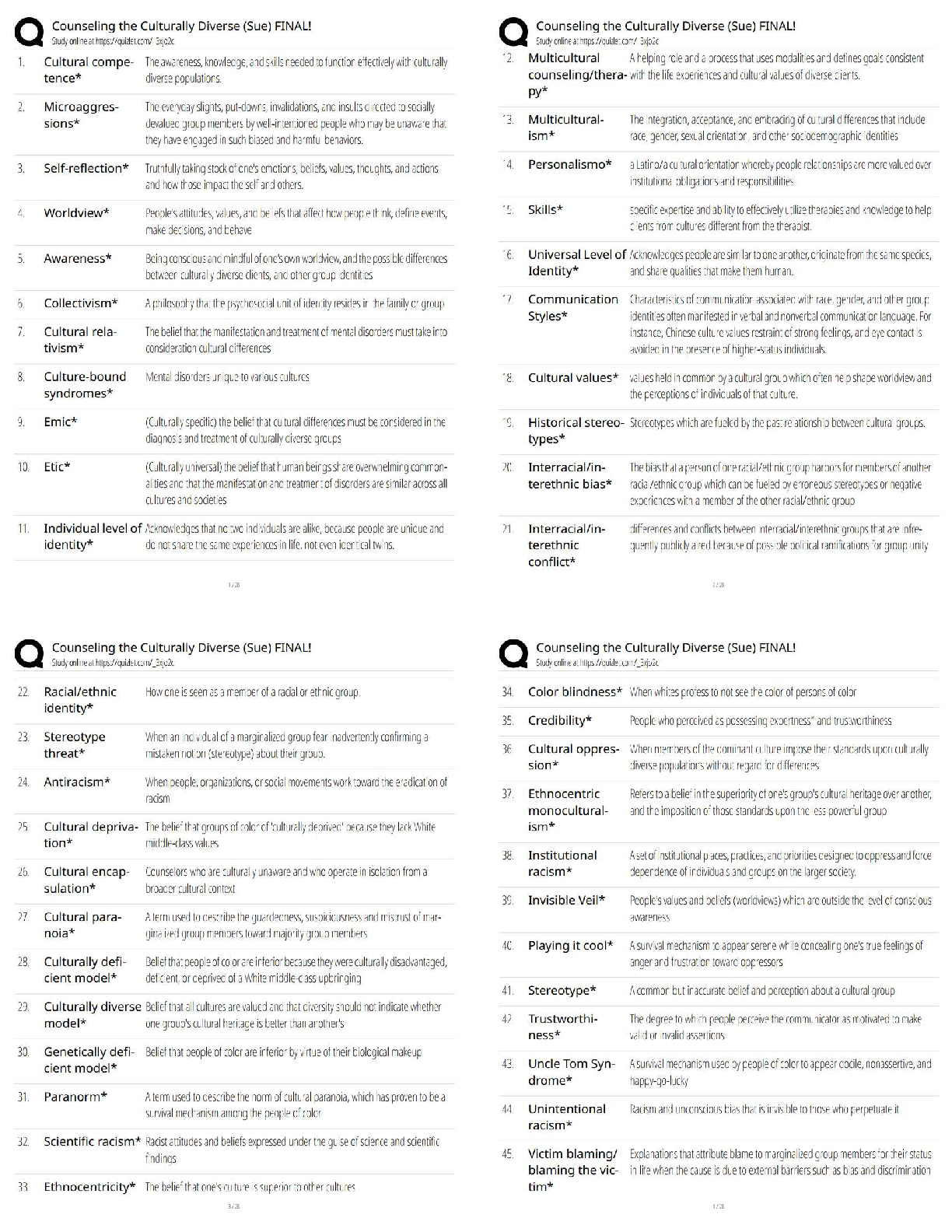
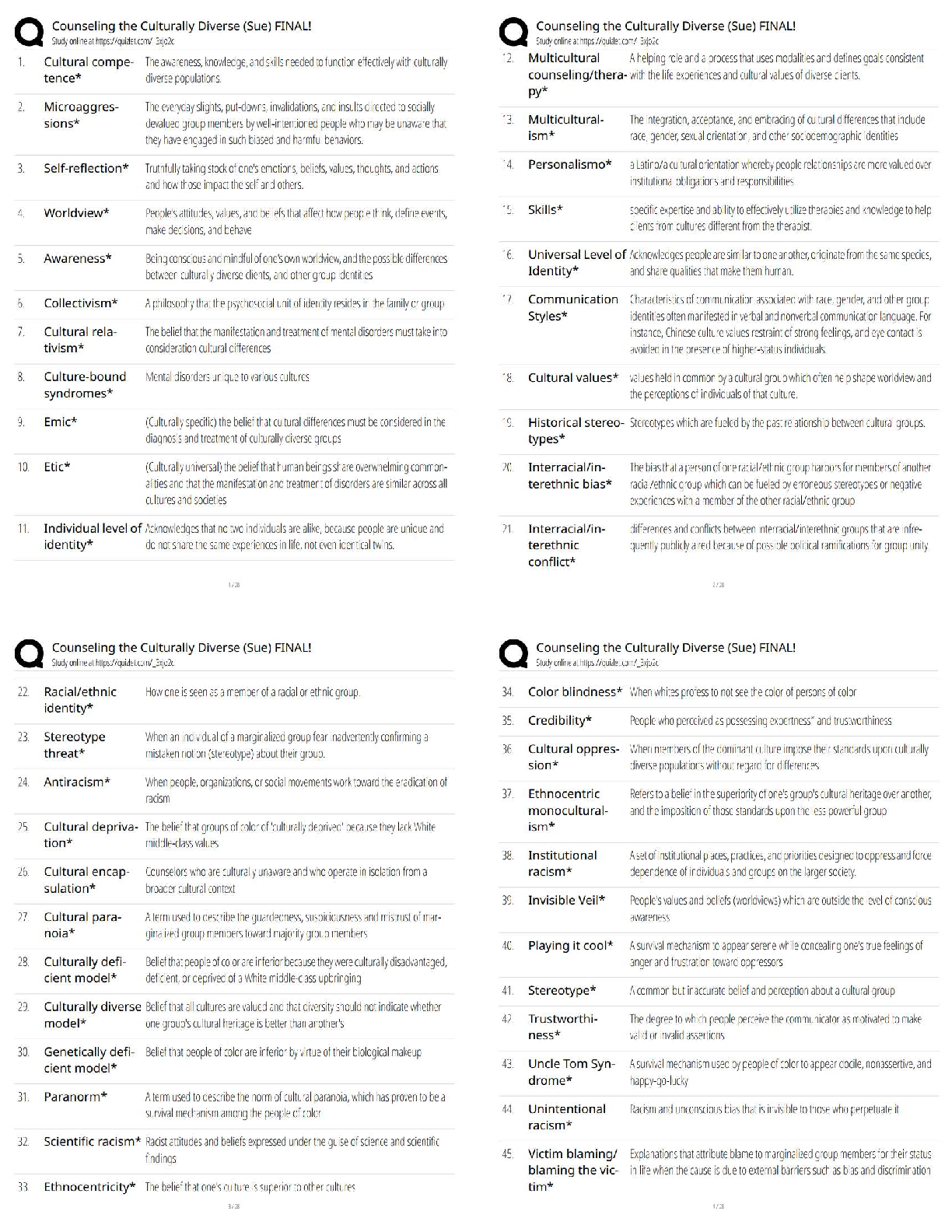
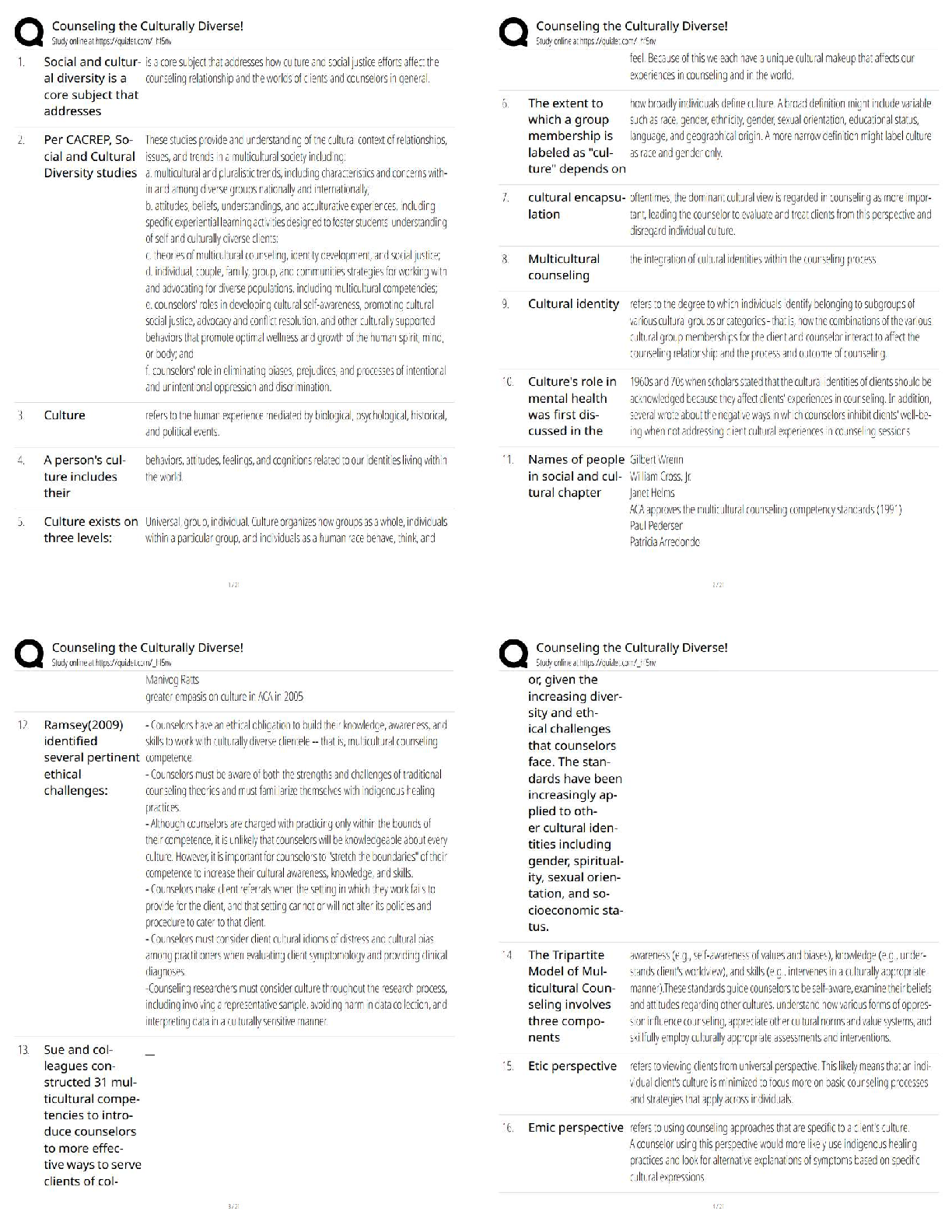
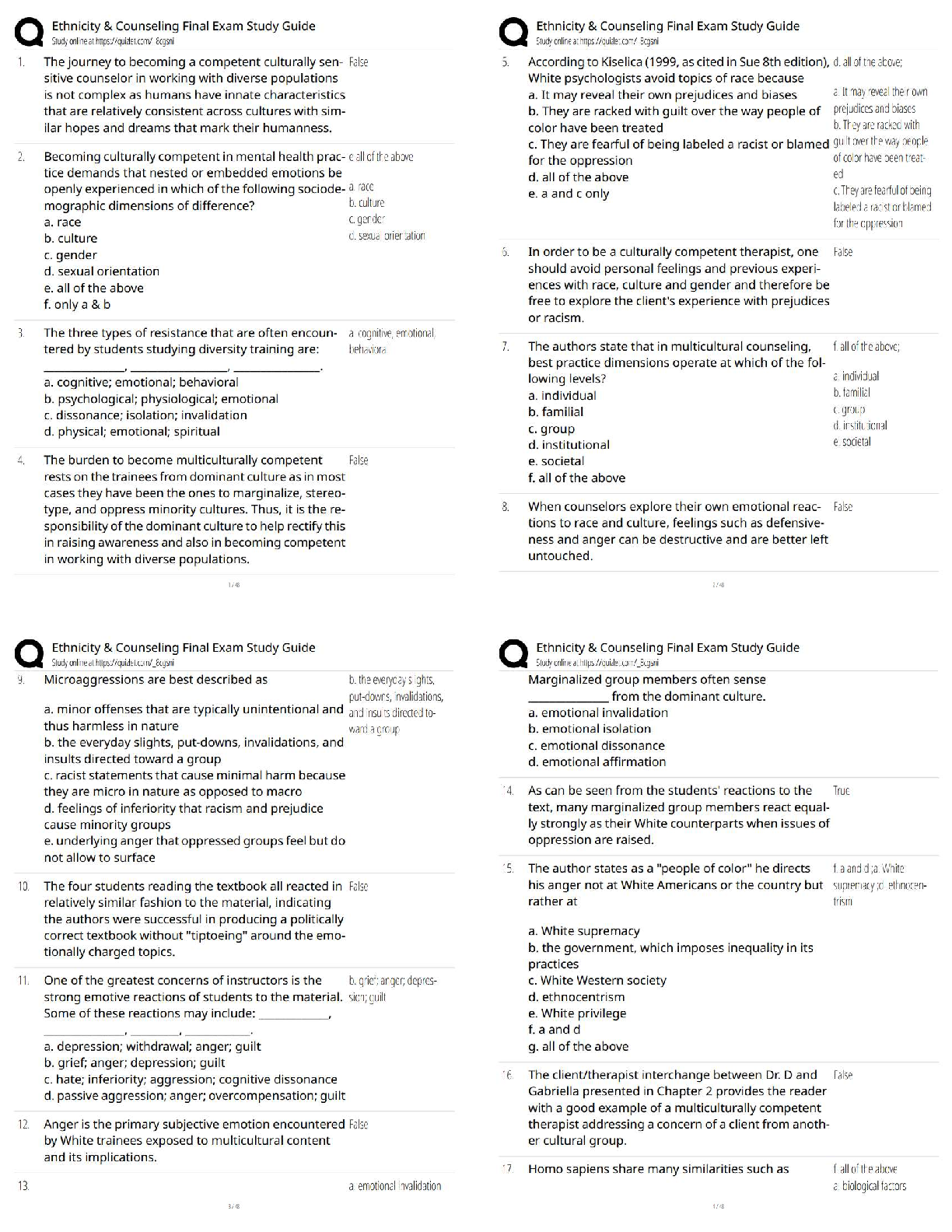
1. Determine the Tf (+ 0.1 ºC) from your plot for Measuring (Cp, cal) by the extrapolation
method shown in Figure 1 of the Pre-Lab. Calculate the heat capacit
...
Boston College
CHEM CH111
Experiment 9 post Lab
1. Determine the Tf (+ 0.1 ºC) from your plot for Measuring (Cp, cal) by the extrapolation
method shown in Figure 1 of the Pre-Lab. Calculate the heat capacity (Cp, cal) of the
calorimeter using Eq. 9. This value is usually small (less than 20 J/Kelvin or 0.020
kJ/Kelvin). It is such a small value that it is of the same order of magnitude as the
experimental error; consequently, the calculation could yield a small negative number. Should
this be the case, simply assign (Cp, cal) the value of 0.0 kJ/Kelvin.
2. Using the plot from Measuring ΔH°rxn(2), determine Tf, and calculate ΔH°rxn(2) using Eq.
10.
Mg was the limiting reactant because it completely dissolved in the HCl solution
Tf= 25.5ºC
Enthalpy of Reaction 2= (Cp, aq) (Va)(Tf-Ta) + (Cp, cal)(Tf-Ta) / (number of moles of
limiting reagent )
3. Treat the data obtained for ΔH°
rxn(3) in a similar manner by determining Tf and calculating
ΔH°
rxn(3) using Eq. 10.
Tf = 23.3ºC
Enthalpy of Reaction 3= (Cp, aq) (Va)(Tf-Ta) + (Cp, cal)(Tf-Ta) / (number of moles of
limiting reagent )
4. Substitute the values you obtained for ΔH°rxn(2) and ΔH°rxn(3), along with the
given value for ΔH°rxn(4), into Eq. 4 to calculate the heat of formation of
magnesium oxide, ΔH°rxn(1) = ΔH°f(MgO).
Questions
1. The concentrations of HCl an NaOH solutions used in the neutralization reaction are
approximately equivalent, yet the volumes of HCl and NaOH solutions used in the reactions
are not equal. Explain why.
2. The flashbulb that Mark is attempting to modify contains 0.41 g of magnesium
metal.
a) Based on the heat of formation of magnesium oxide that you
obtained, how many kJ of energy are released by the flashbulb
contents?
b) Assume the energy released by the reaction is completely absorbed by
the plastic. If 9.8 g of plastic material having a specific heat of 8.2 J/g°C
has been used to manufacture the bulb, what will be the temperature of the
plastic after the flashbulb goes off? Assume the bulb is at room
temperature, 20°C, before ignition.
3. Explain why the final temperature, Tf, must be extrapolated from the plot
(see Figure 1). Why can you not simply use the maximum temperature
recorded?
[Show More]







.png)
.png)
.png)
.png)
.png)