2024 HESI Entrance Exam Guaranteed A+ Actual Questions and Answers, Complete 100%
$ 13

IICRC - Leather Cleaning Technician - Full Test Guide 2024
$ 12

2024 Leadership HESI Exam Guaranteed A+ Actual Questions and Answers, Complete 100%
$ 14

HESI A2 Biology Study Guide 2017.
$ 12

2024 Hesi AP LPN Guaranteed A+ Actual Questions and Answers, Complete 100%
$ 13

Ancient Greek Philosophers Final Milestone Sophia Course
$ 12

VMM2: Competitive Analysis| Business Simulation (D361)WGU TASK 2 (2 versions) Latest 2025/26.
$ 15

HESI MED SURG TEST BANK QUESTIONS AND ANSWERS 100% VERIFIED LATEST 2023/2024
$ 8.5

PMHNP ANCC EXAM 2025 NEW GENERATION QUESTIONS WITH ANSWERS
$ 22

PSC 101 – Exam 1 Study Guide (Harper College, Bobby Summers) with Core Concepts and Practice Questions
$ 11

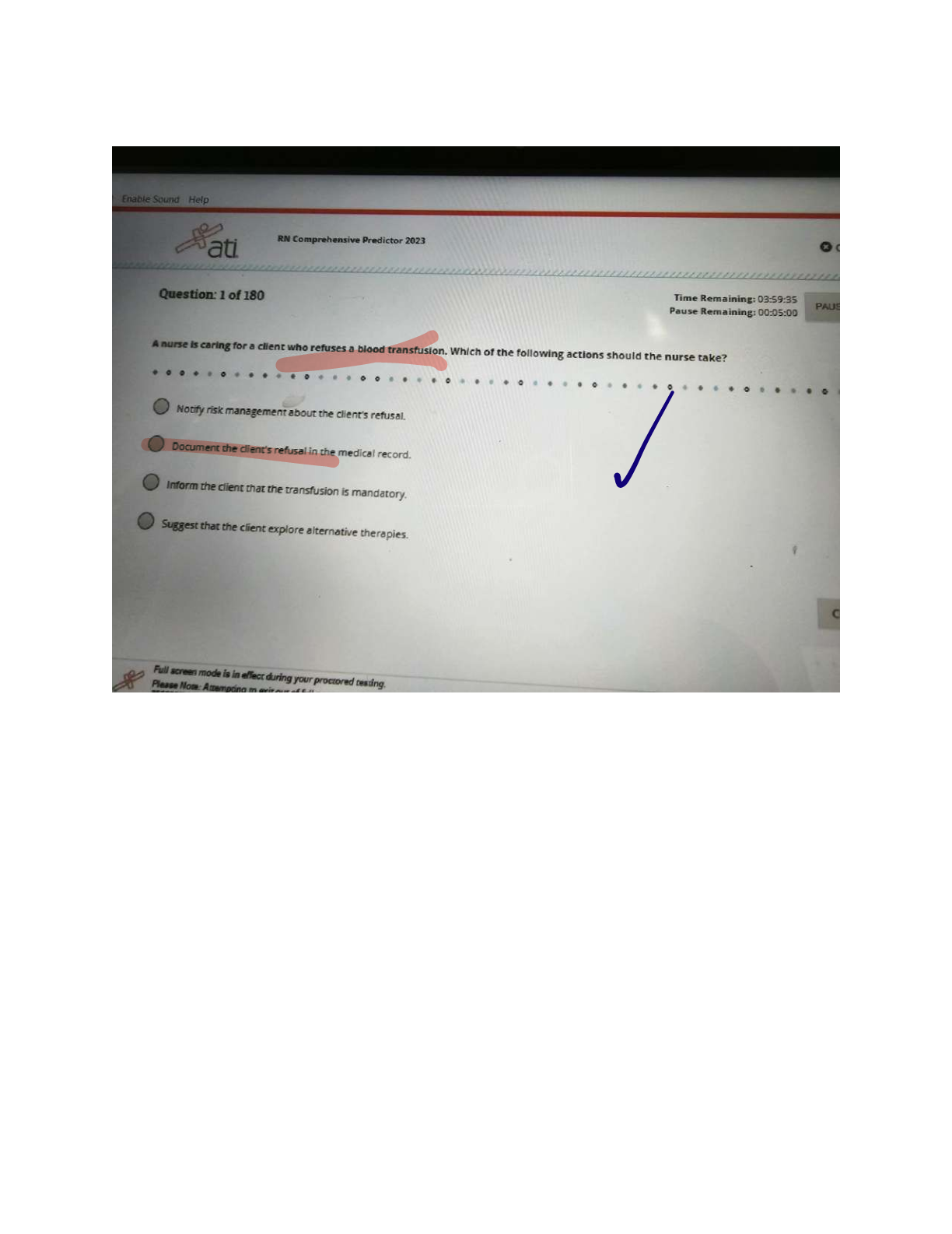
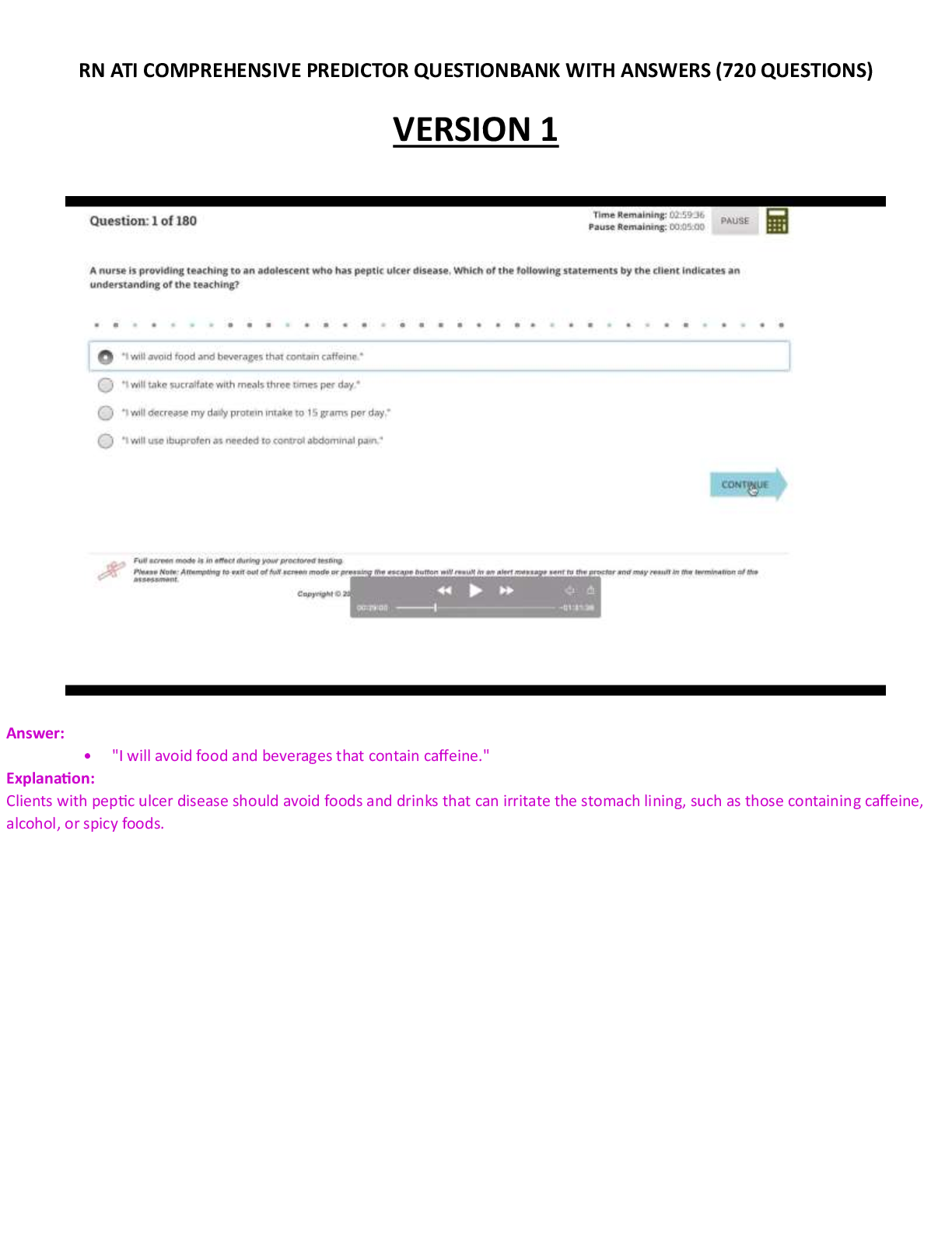
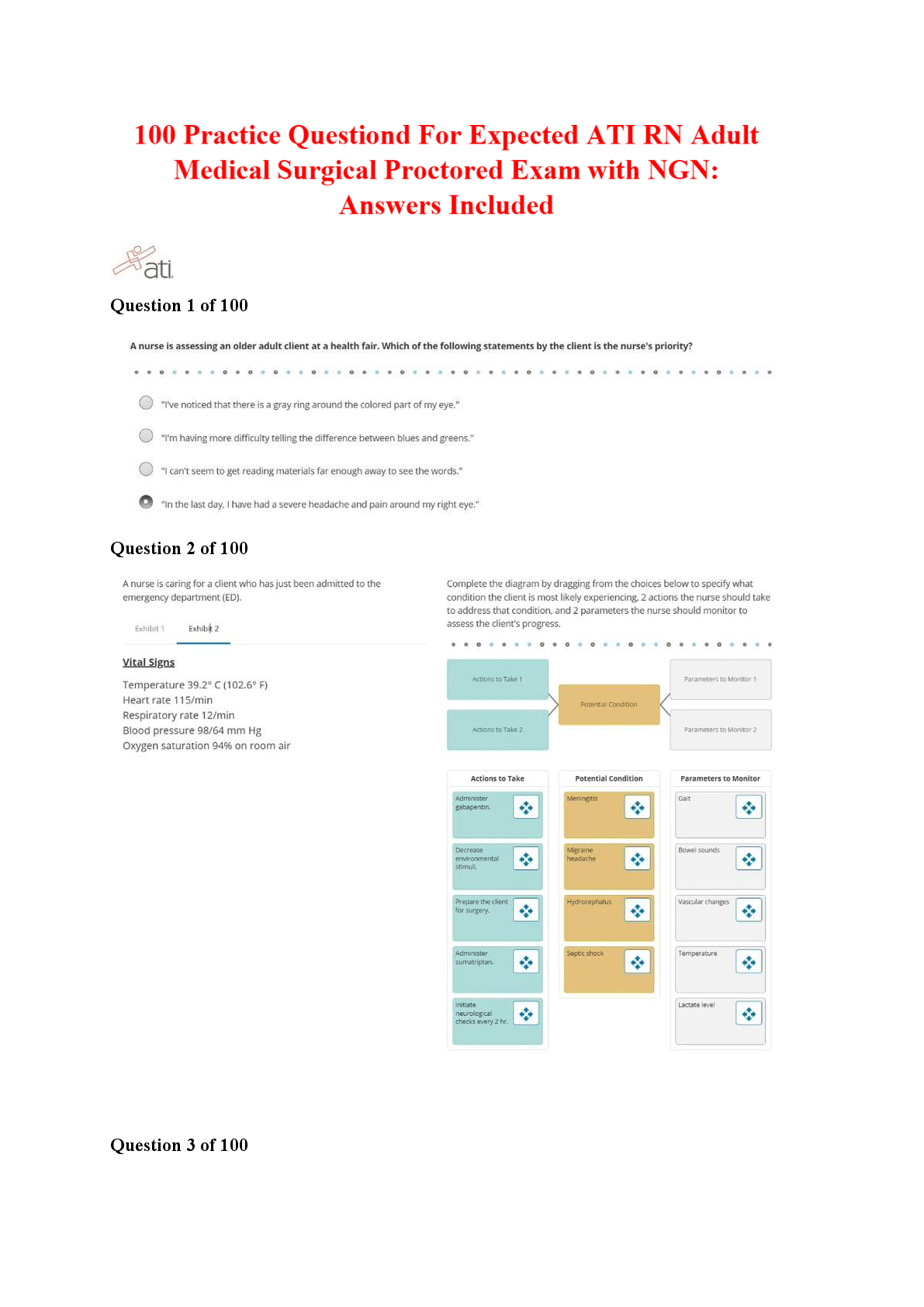
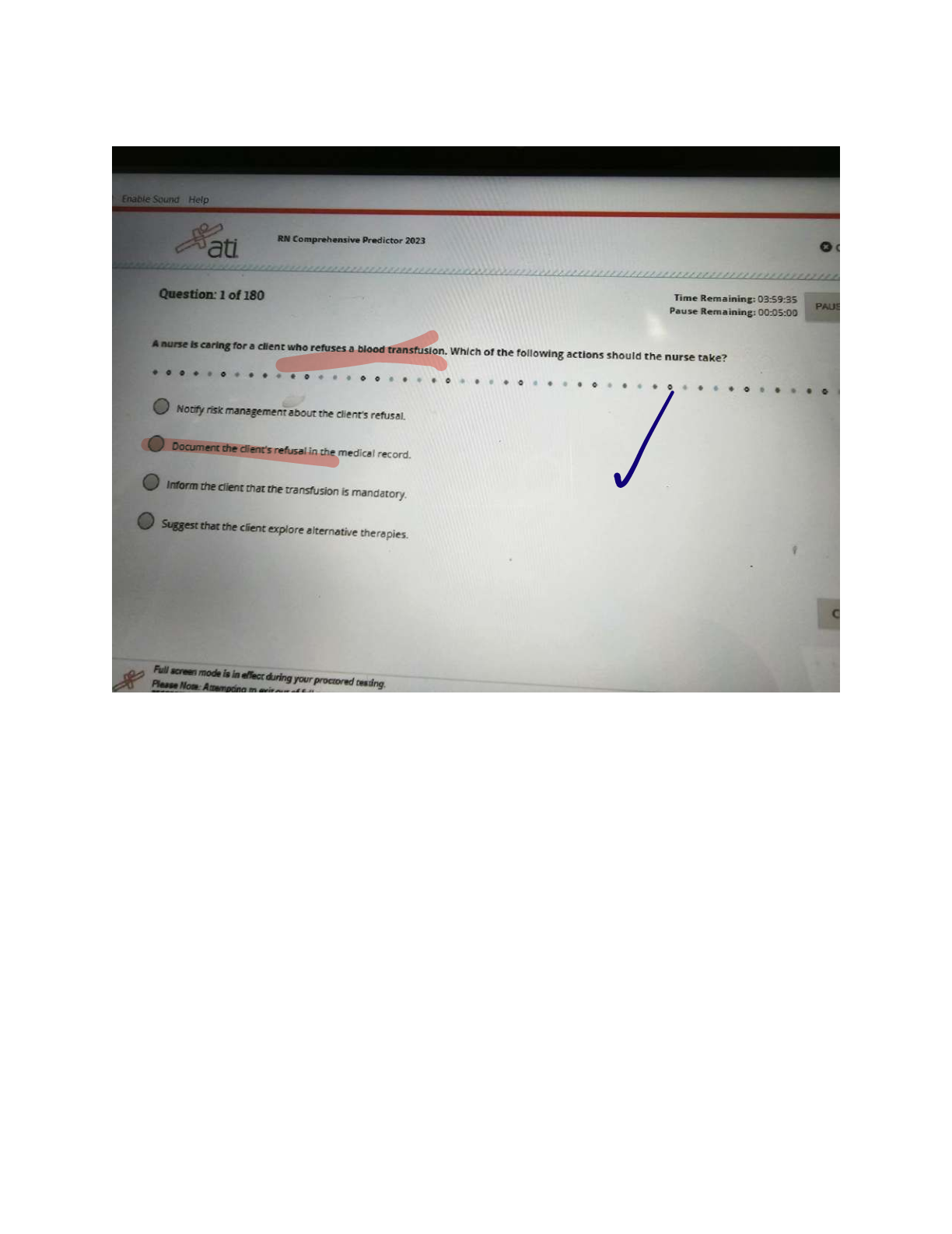
ATI COMPREHENSIVE PREDICTOR EXAM
$ 12.5

Sophia Public Speaking Unit Practice Milestone 4, Updated Revision Study Guide, Correctly Answered Questions, Test bank Questions and Answers with Explanations (latest Update), 100% Correct, Download to Score A
$ 5

CET 345W Materials Testing Laboratory: Compression and Flexure Test of Concrete full lab report.
$ 11

Psychiatric Mental Health Nursing by Mary Townsend (9th Edition, 2017) Test Bank
$ 20
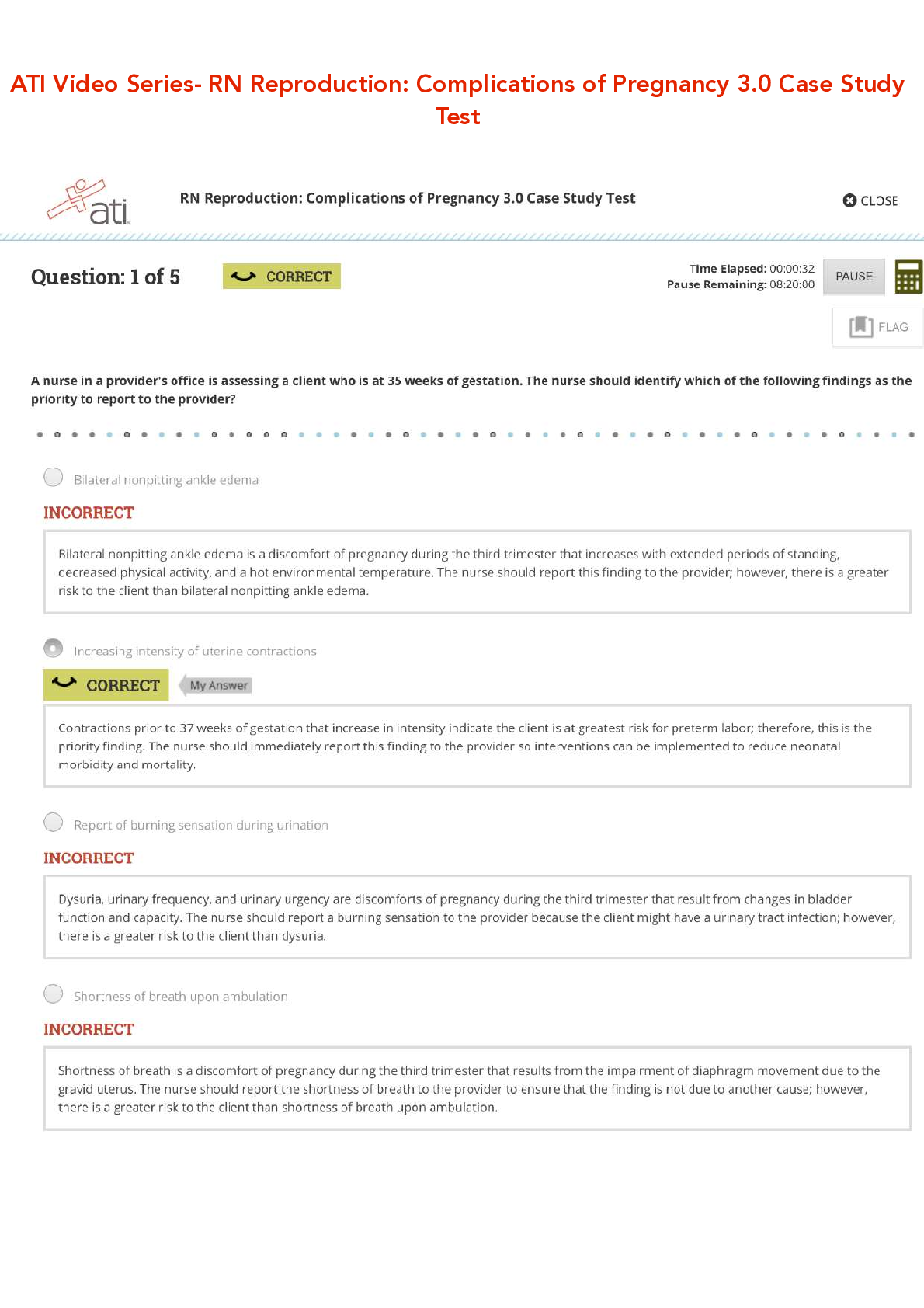
ATI Video Series- RN Reproduction: Complications of Pregnancy 3.0 Case Study Test
$ 7.5
.png)
WGU - C182 – Questions and Answers Latest Update Already Graded A
$ 10

ATI Pharmacology Proctored 2 STUDY GUIDE
$ 15
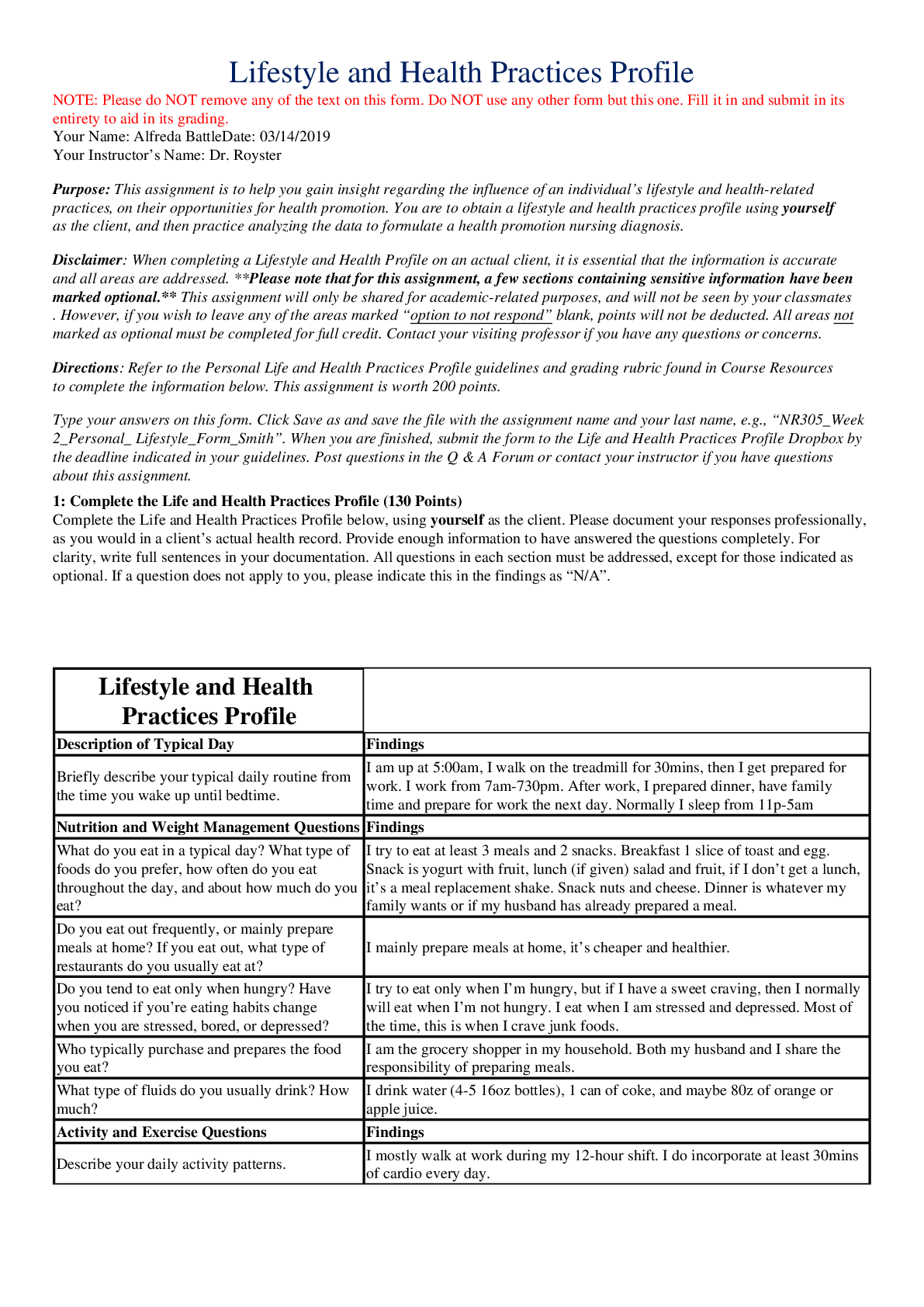
NR 305 WEEK 2 Lifestyle and Health Practices Profile
$ 15
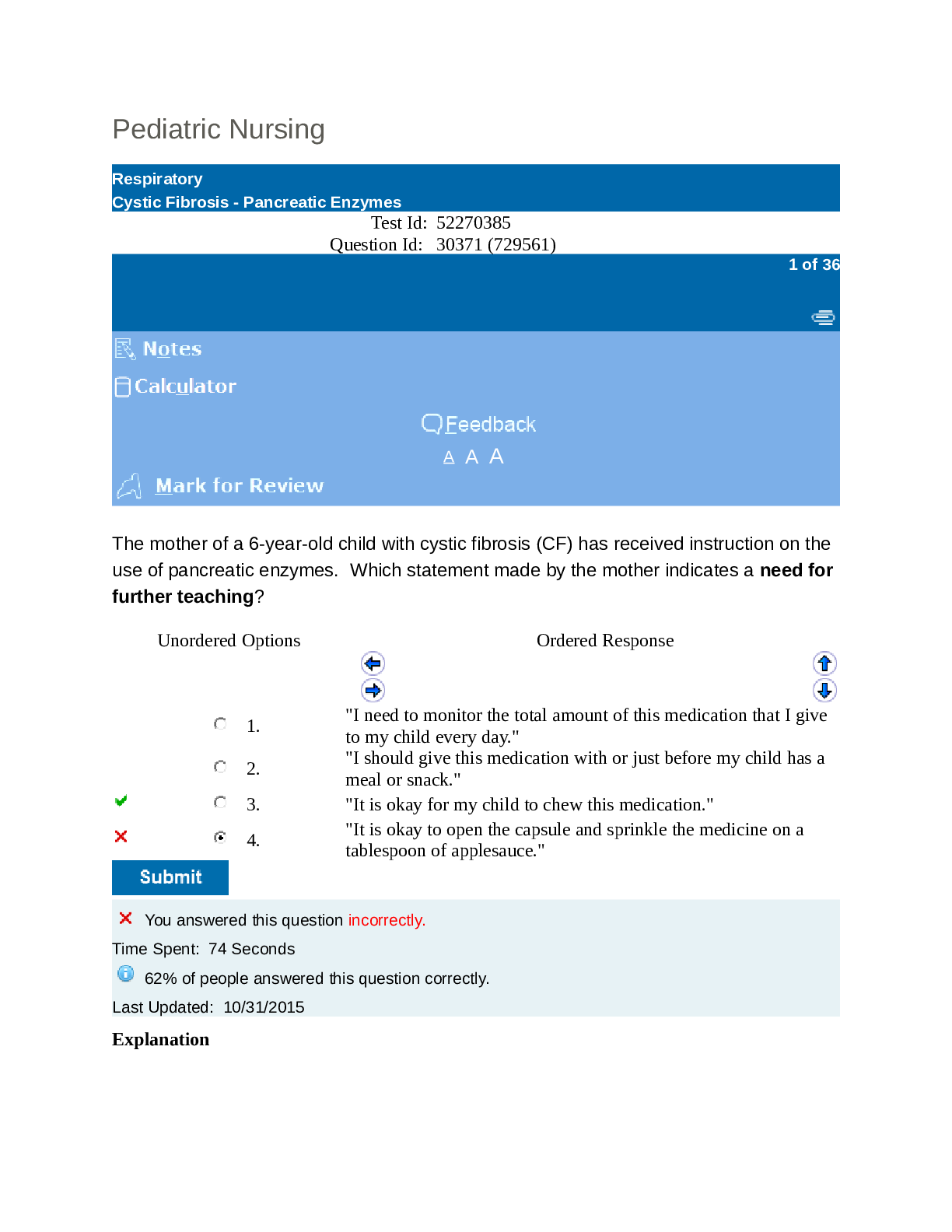
UWorld Pediatric Nursing Test Questions and Answers (Verified 2021).
$ 15.5

NSG 5003 WEEK 7 PATHO QUIZ | GRADED A
$ 10

2024 Hesi Leadership Exit Exam (Actual Version 1) V1 Guaranteed A+ Actual Questions and Answers, Complete 100%
$ 14

HESI Maternity 2 2021
$ 8.5

C H A P T E R 1 4 Calculation of Medication and Intravenous Prescriptions: From Saunders Comprehensive Review for the NCLEX-RN Examination 8th Edition. (Available: https://bit.ly/2HeJuMt ). Contains Practice questions and Answers with the Rationale, Test-Taking Strategy, Level of Cognitive Ability, Client Needs, Integrated Process, Content Area, Health Problem, Priority Concepts and Reference
$ 3

Lehne's Pharmacology for Nursing Care 10th Edition TEST BANK(Chapter 1-110).Questions and Answers with Rationales.Complete Test Bank
$ 25
.png)
PHY 102 Week 4 Exam Solution 80% Guarantee; Complete Solution Guide, Grand Canyon University.
$ 22

NR 599 Informatics Midterm Review Sheet LATEST UPDATE
$ 21
C H A P T E R 11 Nutrition: From Saunders Comprehensive Review for the NCLEX-RN Examination 8th Edition. (Available: https://bit.ly/2HeJuMt ). Contains Practice questions and Answers with the Rationale, Test-Taking Strategy, Level of Cognitive Ability, Client Needs, Integrated Process, Content Area, Health Problem, Priority Concepts and Reference
$ 3

HUM 111 Week 10 Assignment 2020 - Strayer University | HUM111 Week 10 Assignment 2020 - A Grade
$ 15.5

SCIENCE 111: Forces and Motion. All Questions Answered.
$ 12

eBook [PDF] Terraform Cookbook 1st Edition By Kerim Satirli_ Taylor Dolezal
$ 20

eBook Method and Postmethod in Language Teaching 1st Edition By Graham Hall
$ 29

TBS - Phase 1 Exam (Study Guide)



.png)







.png)