MULTIPLE CHOICE
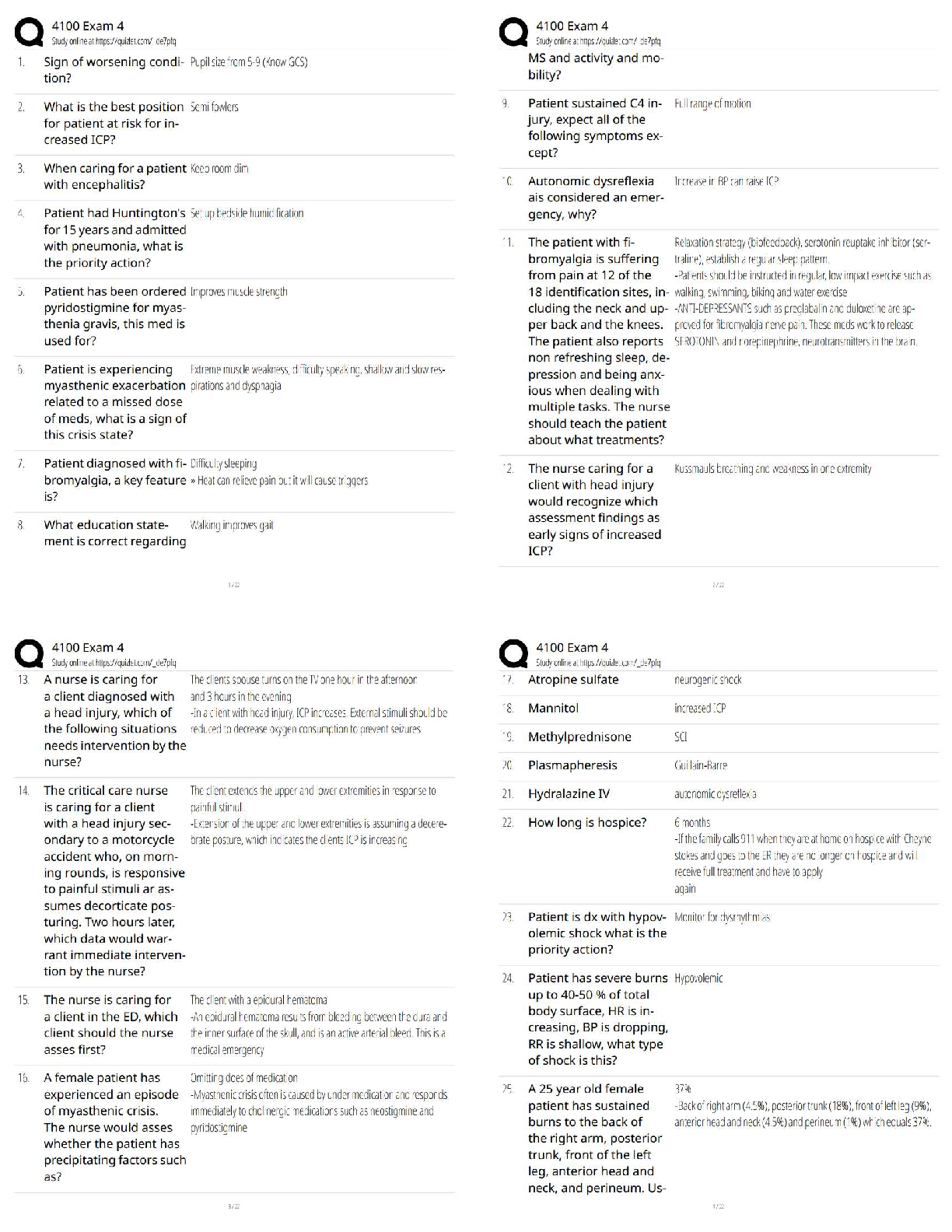
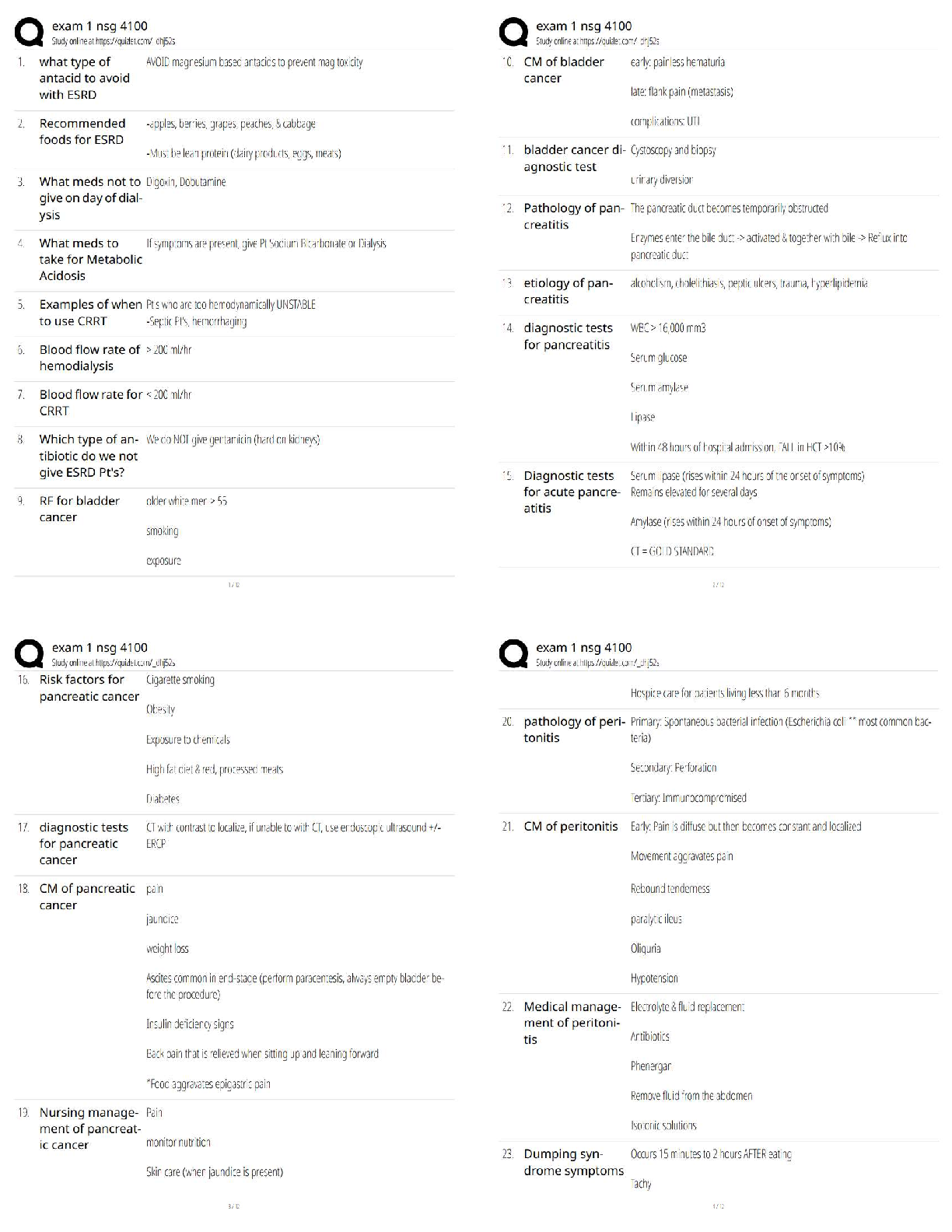
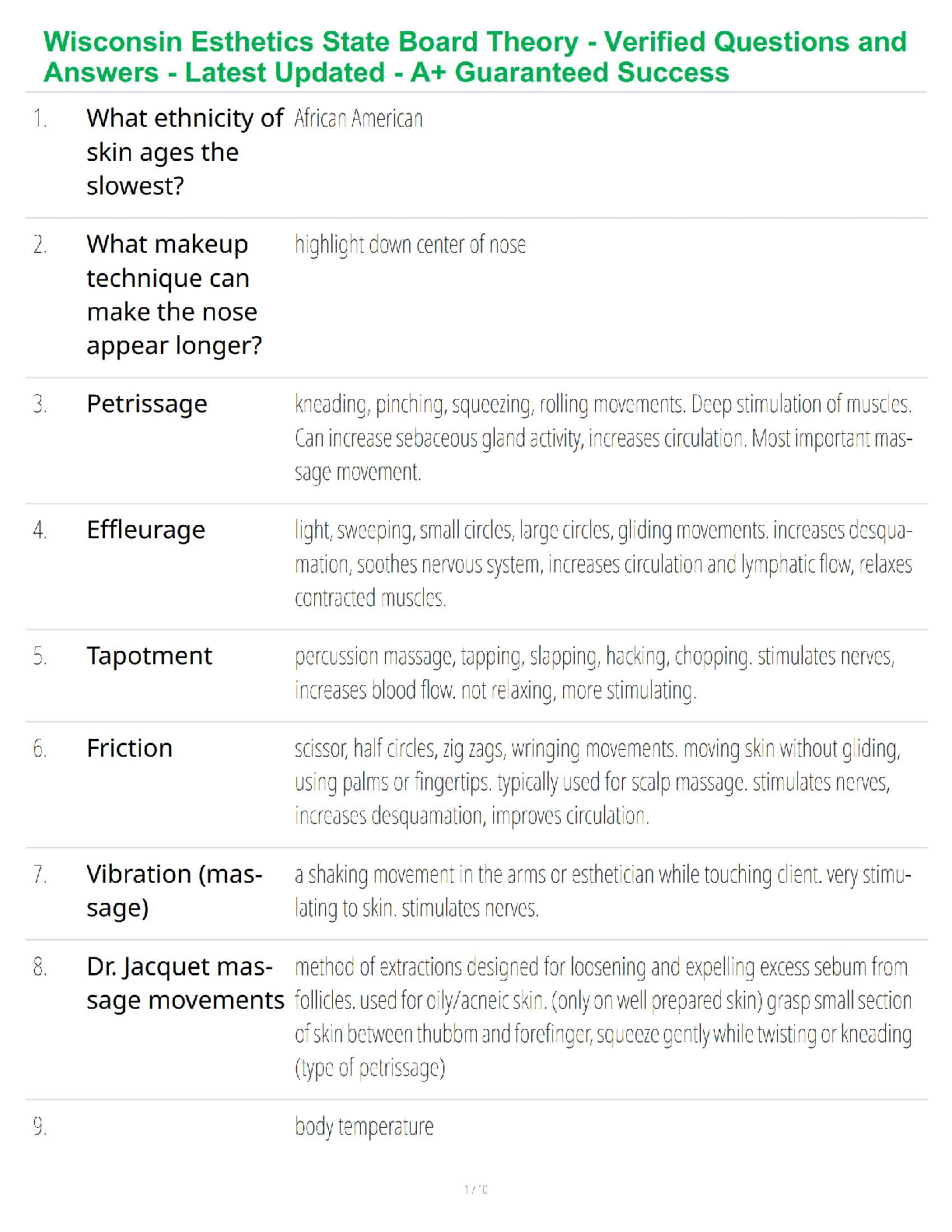
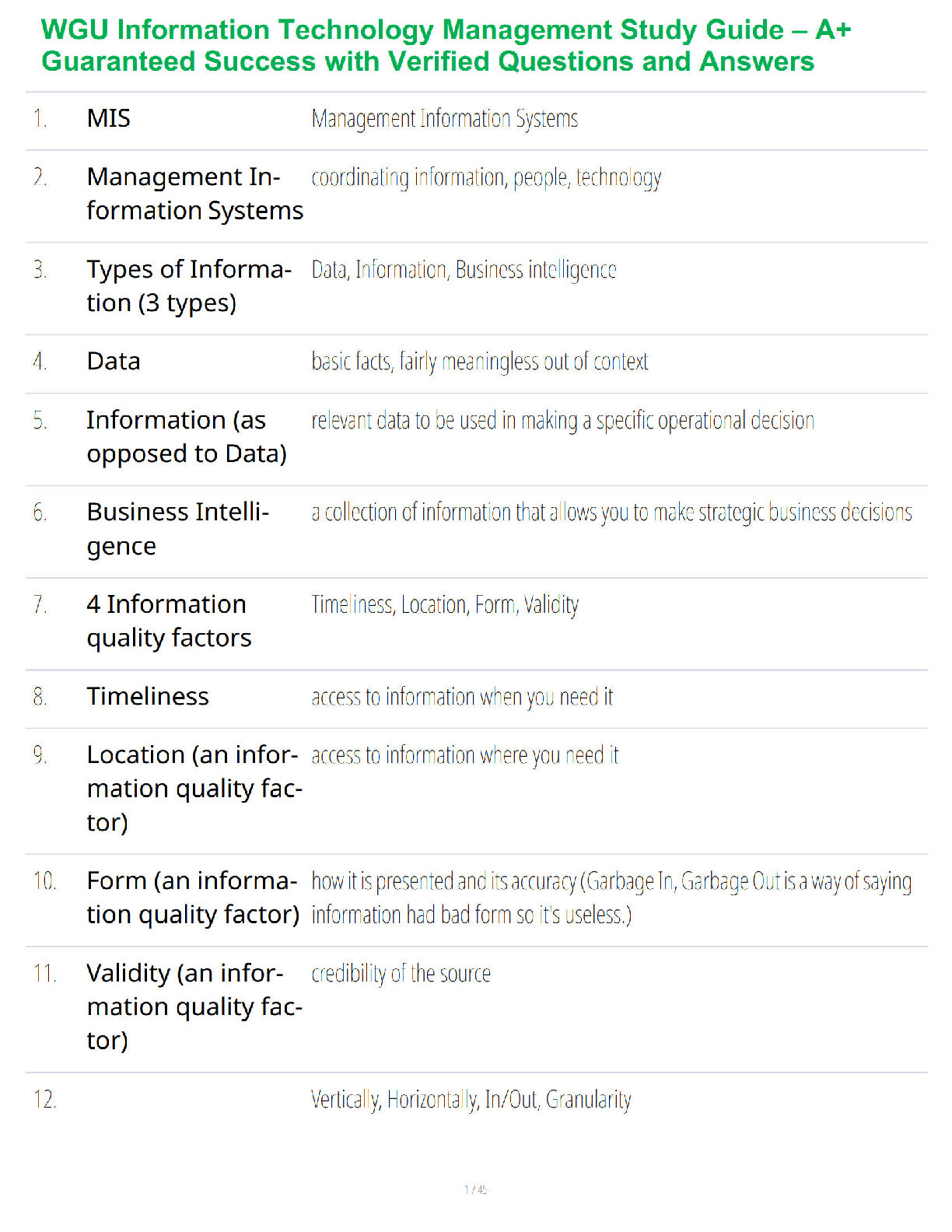
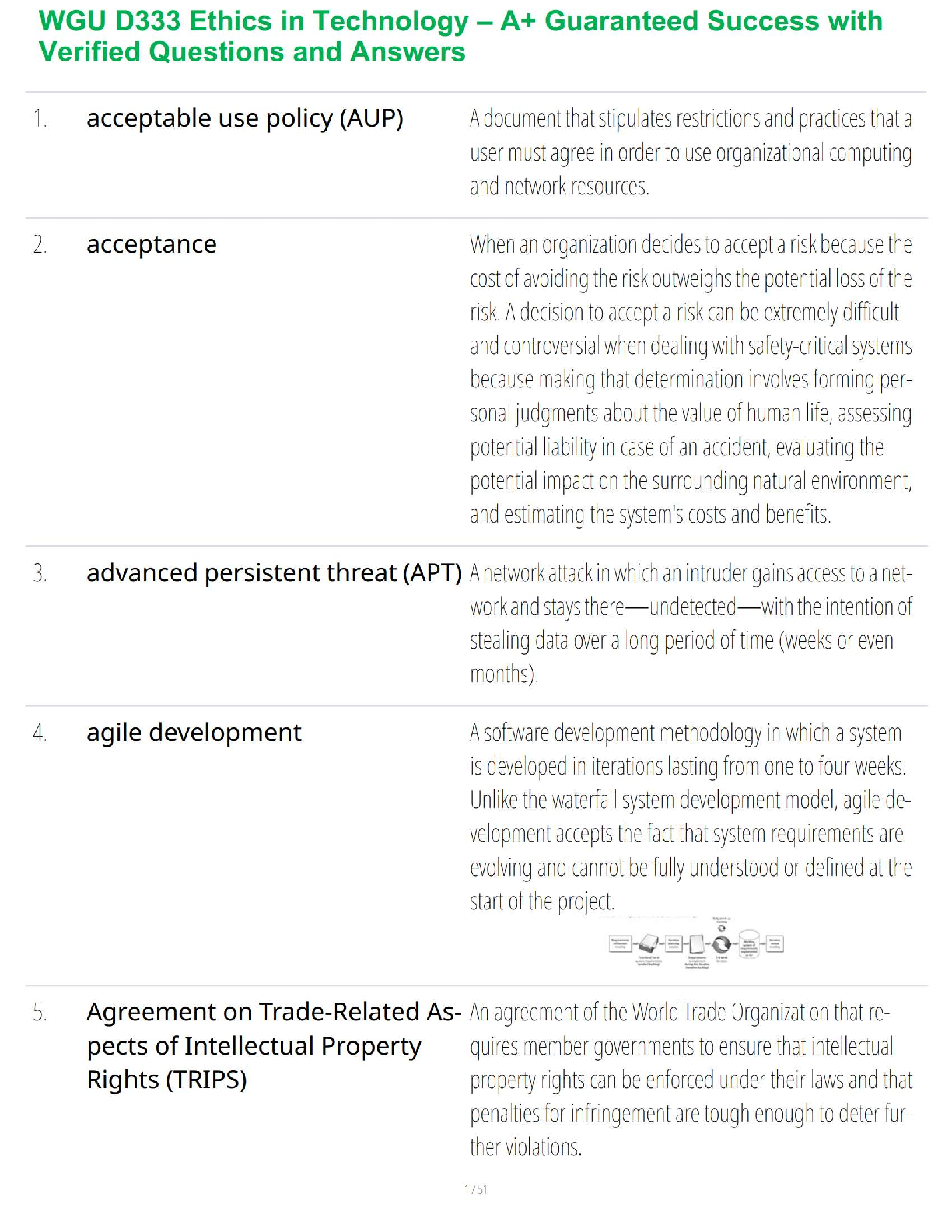
1. If tandem mass spectrometry is combined with a chromatographic separation, what is added to the physical property characterization of the compound being analyzed?
a. Molecular weight
b. Ionizatio
...
MULTIPLE CHOICE
1. If tandem mass spectrometry is combined with a chromatographic separation, what is added to the physical property characterization of the compound being analyzed?
a. Molecular weight
b. Ionization
c. Retention time
d. Ion storage characteristics
2. In which one of the following mass spectrometers does the fragmentation of ions take place after they have been separated by their m/z value in a first stage?
a. In an inductively coupled plasma mass spectrometer
b. In a mass spectrometer interfaced with a gas chromatograph
c. In a quadrupole-trapping spectrometer
d. In a tandem mass spectrometer
3. What is the function of the vacuum system in a mass spectrometer?
a. To produce an ion from a neutral atom or molecule in the initial step
b. To keep ions from colliding during interactions with the magnetic or electric fields
c. To detect, identify, and quantify ion mass in a compound
d. To separate the negative ions from the positive ions with a magnetic field
4. In regard to mass spectrometry, what is a molecular ion?
a. It is a component of a compound in solution.
b. It is the sum of all ions produced displayed as a function of time.
c. It is the unfragmented ion of the original molecule being studied.
d. It is the ion with the highest abundance in the mass spectrum of a compound.
5. How is trapping-type spectrometry different from typical beam-type mass spectrometry?
a. Trapping-type mass spectrometry uses a UV laser to ionize small amounts of matrix and analyte that are directed to the mass analyzer, while beam-types use chemical ionization only.
b. Beam-type mass spectrometry is primarily for complex compound analysis, while trapping-type mass spectrometry is used for elemental analysis only.
c. Trapping-type mass spectrometry uses an ion trap designed to collect ions in three dimensions instead of two dimensions as in a typical beam-type mass spectrometer.
d. Trapping-type mass spectrometers are arranged sequentially in tandem with a gas chromatograph and a “collision cell” placed between the two instruments.
6. You would like to offer a service to the researchers in your institution for identification and quantitative analysis of proteins produced by microorganisms in liquid media using your HPLC-mass spectrometer system. Which one of the ionization techniques is best suited for this analysis?
a. Electrospray
b. Chemical
c. Electron
d. Any of these would work with HPLC-MS.
7. You would like to offer a service to the researchers in your institution for identification and quantification of organic acids produced by microorganisms using your gas chromatograph-mass spectrometer system. Before selecting an ionization technique, you choose to run trial samples with electron and chemical ionization. You notice that the m/z of the acidic ions produced by chemical ionization is higher than the m/z produced using electron ionization. What is the cause of this disparity?
a. Your mass spectrometer has not been standardized correctly for the use of liquid media because both values should be the same.
b. The organic acids are not being separated into separate peaks by the gas chromatograph, producing a faulty reading by the spectrometer’s detector.
c. The fragmented ions of organic acids typically have more than one single charge.
d. Organic acids are not fully fragmented by chemical ionization, yielding a higher mass number.
8. In a mass spectrometer, ion detection is typically accomplished through the use of an electron multiplier. This involves:
a. a densitometer that measures ion effluent and produces a “peak” of activity visible on a computer monitor.
b. a chain of dynodes that “multiplies” the number of electrons to provide a detectable signal.
c. collection of the ion current directly.
d. four parallel electrically conductive rods arranged in a square array that increases electron number.
9. What type of mass spectrometer uses radio frequency–generated fields to confine ions in three dimensions?
a. Quadrupole ion trapping-mass spectrometer
b. Time-of-flight mass spectrometer
c. Tandem mass spectrometer
d. Ion cyclotron resonance mass spectrometer
10. An example of a clinical application of an HPLC coupled to a tandem mass spectrometer would be:
a. determining the presence of trace elements in blood.
b. screening and confirming the presence of inborn errors of metabolism.
c. identifying specific protein.
d. quantifying drugs of abuse.
11. In a mass spectrometer, the ion with the highest abundance in the mass spectrum that is assigned a relative abundance of 100% is referred to as the:
a. base peak.
b. ion trap.
c. ionic chromatogram.
d. time-of-flight ion.
12. The soft ionization technique that uses a UV-absorbing compound upon which the analyte of interest is placed and eventually vaporized into a plume of ions directed into the mass analyzer is:
a. atmospheric pressure photoionization.
b. electrospray ionization.
c. matrix-assisted laser desorption ionization.
d. inductively coupled plasma ionization.
13. Because of the ability to identify and quantify proteins in a complex mixture, mass spectrometry combined with separation methods is an excellent analytical tool used specifically in the field of:
a. genomic research.
b. inborn errors of metabolism.
c. trace metal analysis.
d. proteomics.
[Show More]








.png)
.png)
.png)
.png)