BioChemistry > QUESTIONS & ANSWERS > BCHE5080. bIOcHEMISTRY: HW 2 - Answers for Protein Purification 100% Correct. (All)
BCHE5080. bIOcHEMISTRY: HW 2 - Answers for Protein Purification 100% Correct.
Document Content and Description Below
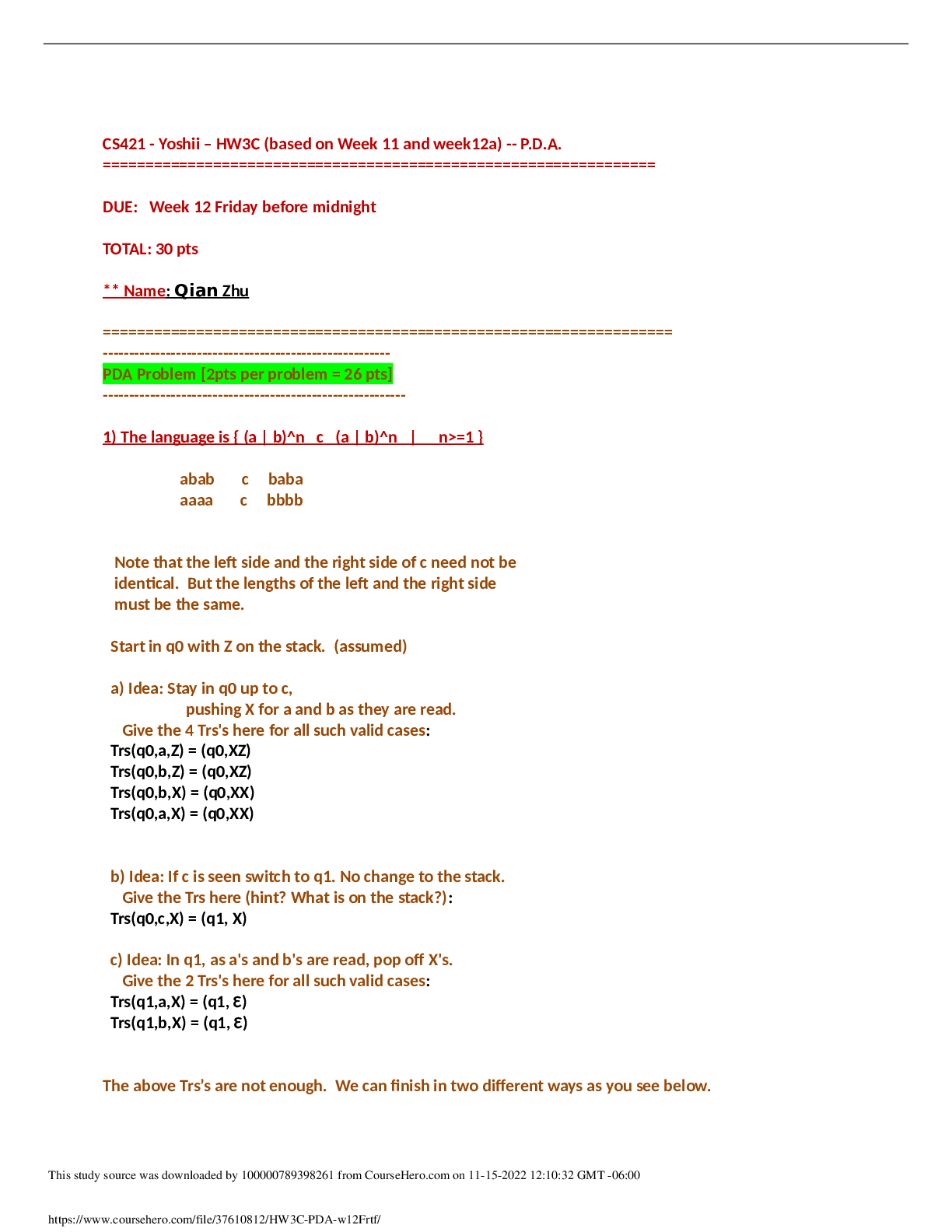
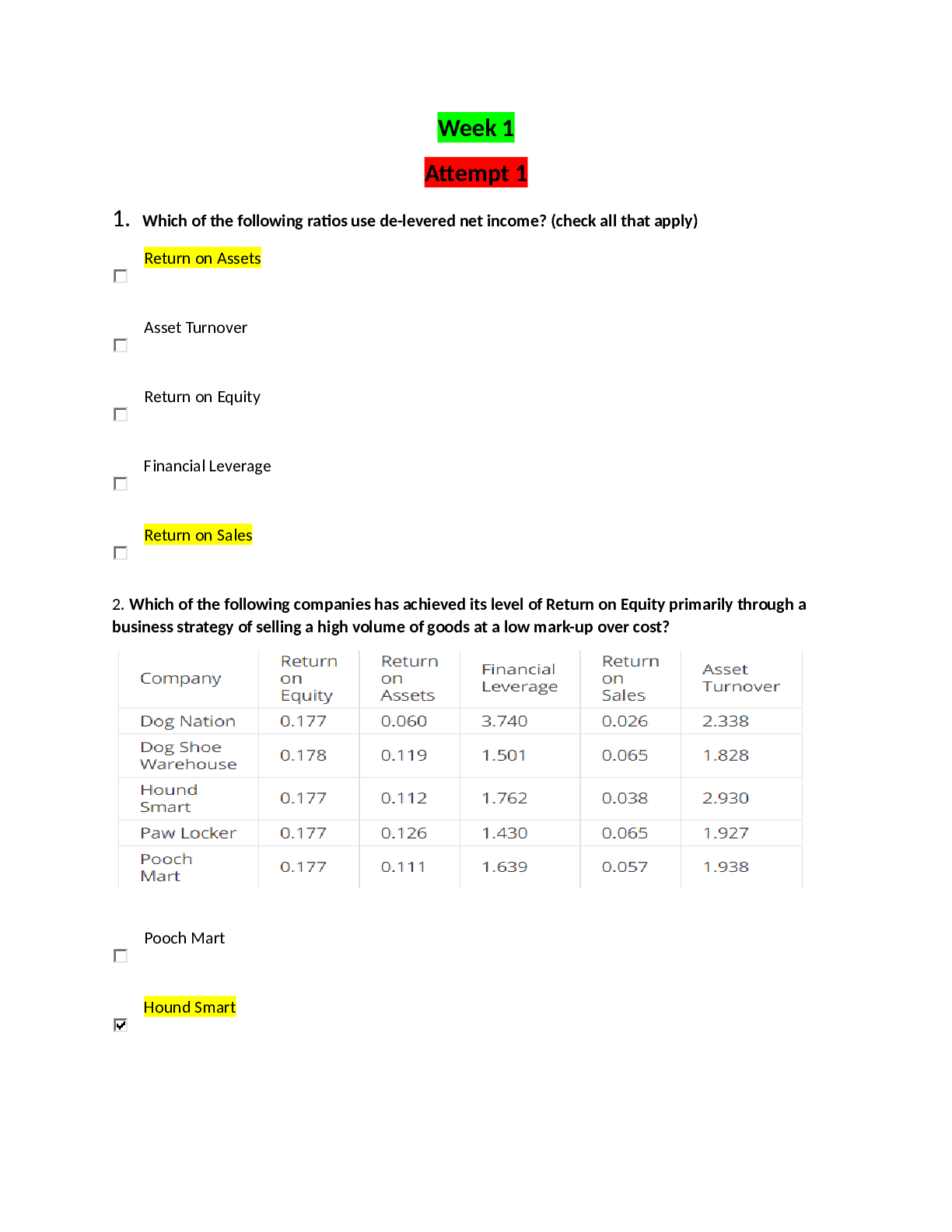
Make a reading outline for those sections in the class slides/notes and your text that discuss biochemical techniques (Section 3.3 and p. 179 in Nelson/Cox or Section 5.2 in Garrett/Grisham). Pay atte... ntion to the following techniques: • Purification • Ammonium sulfate precipitation • Chromatography - Gel filtration/size exclusion (same thing, just two different names) - Ion exchange - Hydrophobic interaction - Affinity chromatography • Detection • Absorption spectroscopy • Gel electrophoresis (Determining the MW and number of subunits in a protein). - SDS and native gels - Isoelectric focusing • Protein assays • Immunological methods (These methods use antibodies). - ELISA - Western immunoblotting To get more at home with some of these techniques and how they are displayed and represented in papers you can look at some of the animations available online. GE healthcare has several animations covering different types of chromatography: http://proteins.gelifesciences.com/knowledge-library/video-hub/ or https://www.youtube.com/user/gelifesciences. Assay of Proteins Total (non-specific) Protein Determination These assays are performed to determine or estimate the amount of all protein in a given sample. 1. UV Absorption - Proteins have a characteristic absorption between 275-280 nm due to the presence of tyrosine and tryptophan residues. 2. Colorimetric Reactions - Several reagents have been developed that bind or complex with proteins to form colored products that can be measured spectrophotometrically Specific Protein Determinations For any purification or characterization of a specific protein, some method must be found to quantitatively detect its presence. 1. Enzyme Activity - As we will see, enzymes are proteins with catalytic activity. An assay for an enzyme that catalyzes a reaction with a readily detectable product can be easily developed. 2. Binding Assays - Some proteins, such as receptors, are often assayed because of their ability to bind to specific ligands. 4. Bioassays - Many proteins, like hormones, have specific biological effects. The effects on a standard tissue, cell, or whole organism can be observed and often quantified Purification of Methyl-coenzyme M Reductase from Methanothermobacter marburgensis To put all the theory about the difference purification steps and techniques in context we will examine the purification procedure of methyl-coenzyme M reductase. This is an enzyme found in methanogenic Archaea that catalyzes the reaction: Membranes vs. cytosol When a purification is performed for the first time, the first step will always be to determine in what part of the cell the activity can be found. After the cells are broken the protein solution is ‘spun-down’ at high speed in a centrifuge. This will cause all the membrane fragments to form a pellet at the bottom. After centrifugation the cytosol can be decanted and kept in a new tube. Buffer can be added to the old tube to resuspend the pellet with the membrane fragments. Now both solutions can be tested for the desired activity. In the case of methyl-coenzyme M reductase it was found that the activity is present in the cytosolic fraction. Therefore the cytosol is collected (2: ‘cell extract’) and used in the next purification step. Ion-exchange chromatography Ion-exchange chromatography is based on the attraction of charged proteins to oppositely charged beads present in a chromatography column Why In order to understand biomolecules, biochemists often need to isolate these molecules from the biological medium and, once isolated, to characterize them chemically. Many techniques are available to accomplish these tasks. In addition, biochemists have found ways to chemically synthesize some biomolecules. In understanding these techniques and procedures you will gain a better understanding of the nature of biomolecules, their functions, and how biochemists manipulate them to gain additional understanding of them. Outcomes 1. Describe the molecular basis for some techniques used to isolate and characterize biomolecules in general, and proteins and amino acids specifically. 2. Select proper techniques or sequence of techniques to accomplish a given isolation or biomolecular characterization. 3. Use critical thinking and problem solving skills to identify necessary tools to elucidate aspects of protein structure. Critical Thinking Questions 1. Which technique(s) could you utilize to separate two proteins that (a) differ greatly by size, (b) differ by pl, and (c) that have similar physical characteristics (e.g. size and pl), but have very different functional characteristics? In each case briefly describe how the separation would be accomplished. 2. On the computer go to the link https://www.youtube.com/watch?v=IWZN_G_pC8U and watch the animation. In this example both protein samples contain 3 bands. Does that mean that we have 3 different proteins in each fraction or are there other possibilities? Discuss the different options (if any). 3. Imagine protein with subunits. This 100 kDa protein has two subunits (70 kDa and 30 kDa) joined by two disulfide bonds. Draw the gel electrophoresis pattern for SDS-PAGE with a lane that includes 2-mercaptoethanol (2-ME) and one lane without 2-ME. Be sure to include a molecular weight (MW) marker lane. Note: 2-ME is one of two commonly used reducing agents. The other is DTT (dithiothreitol). Explain your drawing. Are subunits always kept together with disulfide bonds? 4. Both SDS-PAGE and gel filtration chromatography are used to separate proteins based on size. On the computer go to the link https://www.youtube.com/watch?v=oV5VB5kO3tQ and watch the animation. Imagine you have a mixture of proteins: (b) If your experimental goal is to collect each intact protein for further analysis, would gel filtration or SDS-PAGE be preferable? Why? 5. Watch the animation on ion exchange chromatography on the GE healthcare site: http://proteins.gelifesciences.com/knowledge-library/video-hub/ or https://www.youtube.com/user/gelifesciences. A graph is generated during the purification procedure, the so-called elution profile. Redraw the graph and label all the axes. Explain how such a graph is obtained in the lab. Refer to the labels of the x- and y-axes to help determine what data were collected. What is being detected in the broad gray peak? What is being detected in the colored peaks? How would a scientist wishing to isolate a particular protein use the information given in the plot? 6. What techniques can be used to determine the amount of a specific protein in a sample? What characteristic(s) of the protein does each technique take advantage of? 7 An isoelectric focusing experiment with a protein sample shows that there are 3 protein present with a pI of 3 (protein A), 7 (protein B) and 9 (protein C). Since the focusing experiment can only be done with very small amounts of protein an ion exchange column purification step is designed to separate the three proteins. Describe how you would design this experiment: What column will you use (cation or anion exchange)? What will be the pH of the buffer used in the procedure? Draw the expected elution profile for your column. 8. A biochemist discovers and purifies a new enzyme, generating the purification table below. This is another example of a more realistic set of steps when trying to purify an enzyme for the first time. This includes a lot of trial-and-error. A good examination of the purification table will help to remove unnecessary steps for future purifications. (a) From the information given in the table, calculate the specific activity of the enzyme after each purification procedure. Why do we calculate this? (b) Which of the purification procedures used for this enzyme is most effective (i.e., gives the greatest relative increase in purity)? (c) Which of the purification procedures is least effective? (d) Is there any indication based on the results shown in the table that the enzyme after step 6 is now pure? What else could be done to estimate the purity of the enzyme preparation? (e) Would you advice the biochemist to perform the purification the same way the next time he performs this or would you recommend any changes in the procedure. If any, what changes would you recommend? 9. Refer to the purification table for methyl-coenzyme M reductase. Describe and discuss the different trends in the different columns of the table. For example, the total amount of protein is going down. Is that a good thing or a bad thing? 10. Shown in this document is a very polished purification procedure for methyl-coenzyme M reductase. The first time this enzyme was purified, however, the information provided would not have been enough to decide it was pure at this stage. Discuss, why that is and what other purification steps and or procedures would have been performed to conclude that the enzyme was pure after the Q-sepharose step. After you did this, the professor will provide you with data for an additional purification step. Discuss this new data. Does it confirm your proposal? [Show More]
Last updated: 2 years ago
Preview 1 out of 17 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 17, 2020
Number of pages
17
Written in
Additional information
This document has been written for:
Uploaded
Mar 17, 2020
Downloads
0
Views
276




.png)
.png)
.png)
.png)
.png)
.png)
.png)

