Chemistry > QUESTIONS & ANSWERS > Introduction to Organic Chemistry. Questions and Answers. (All)
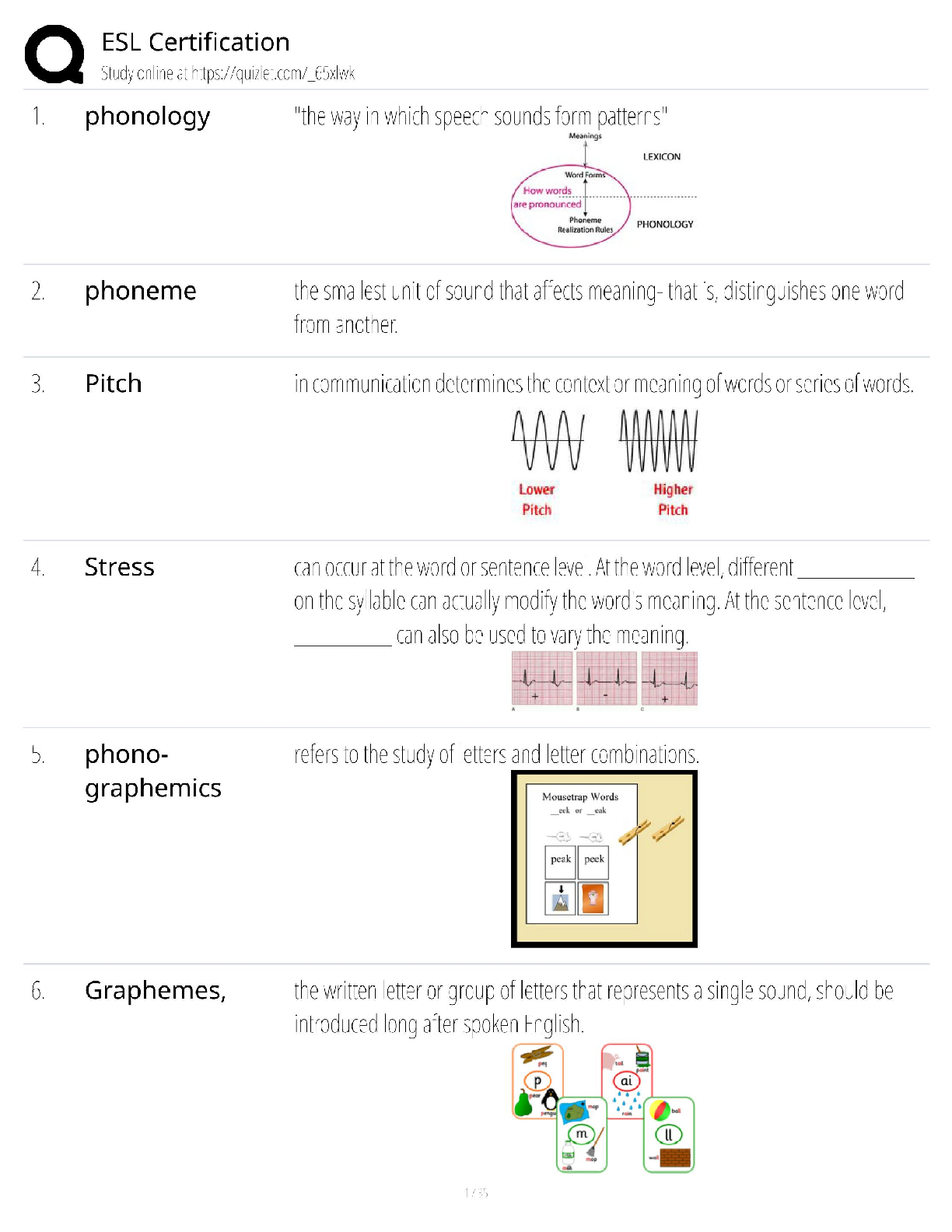
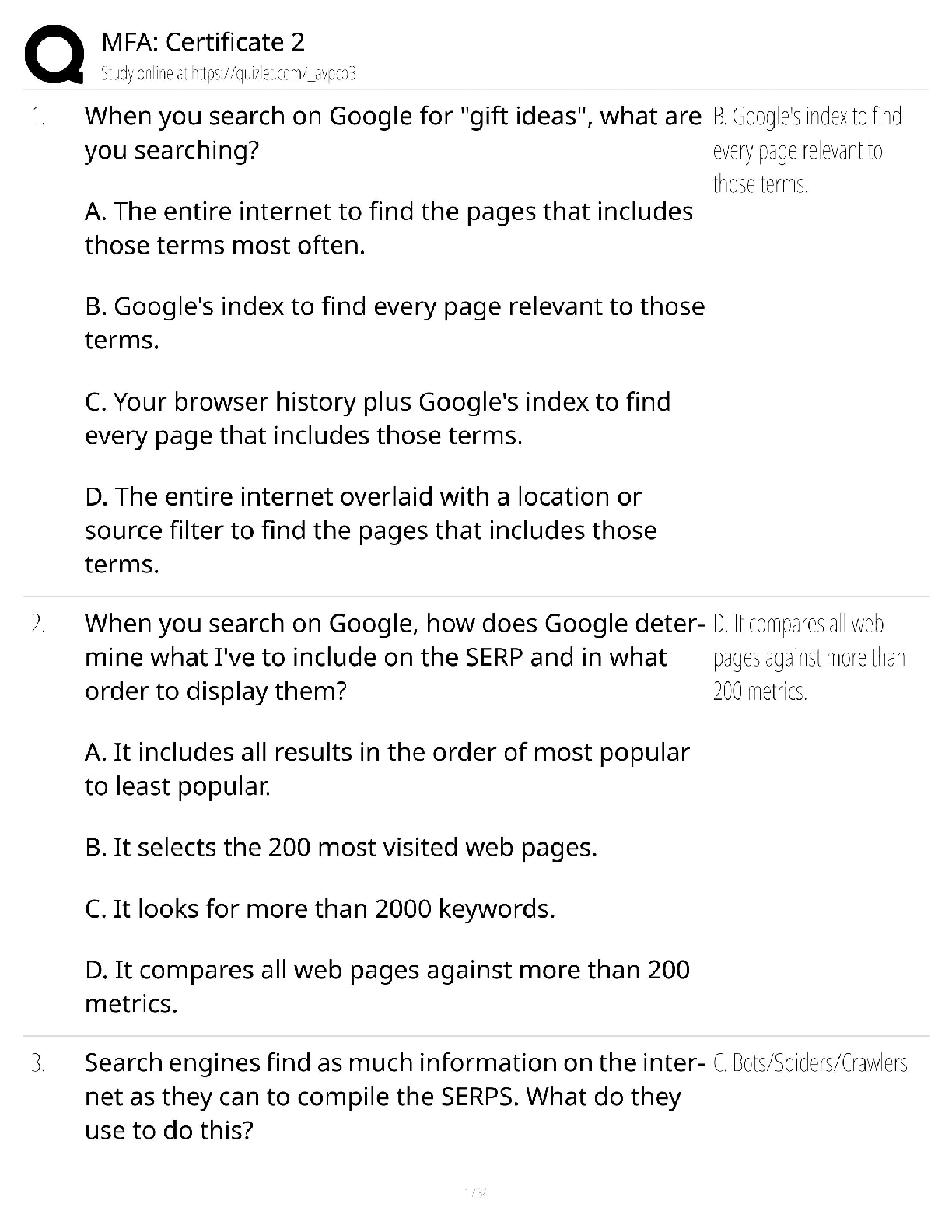
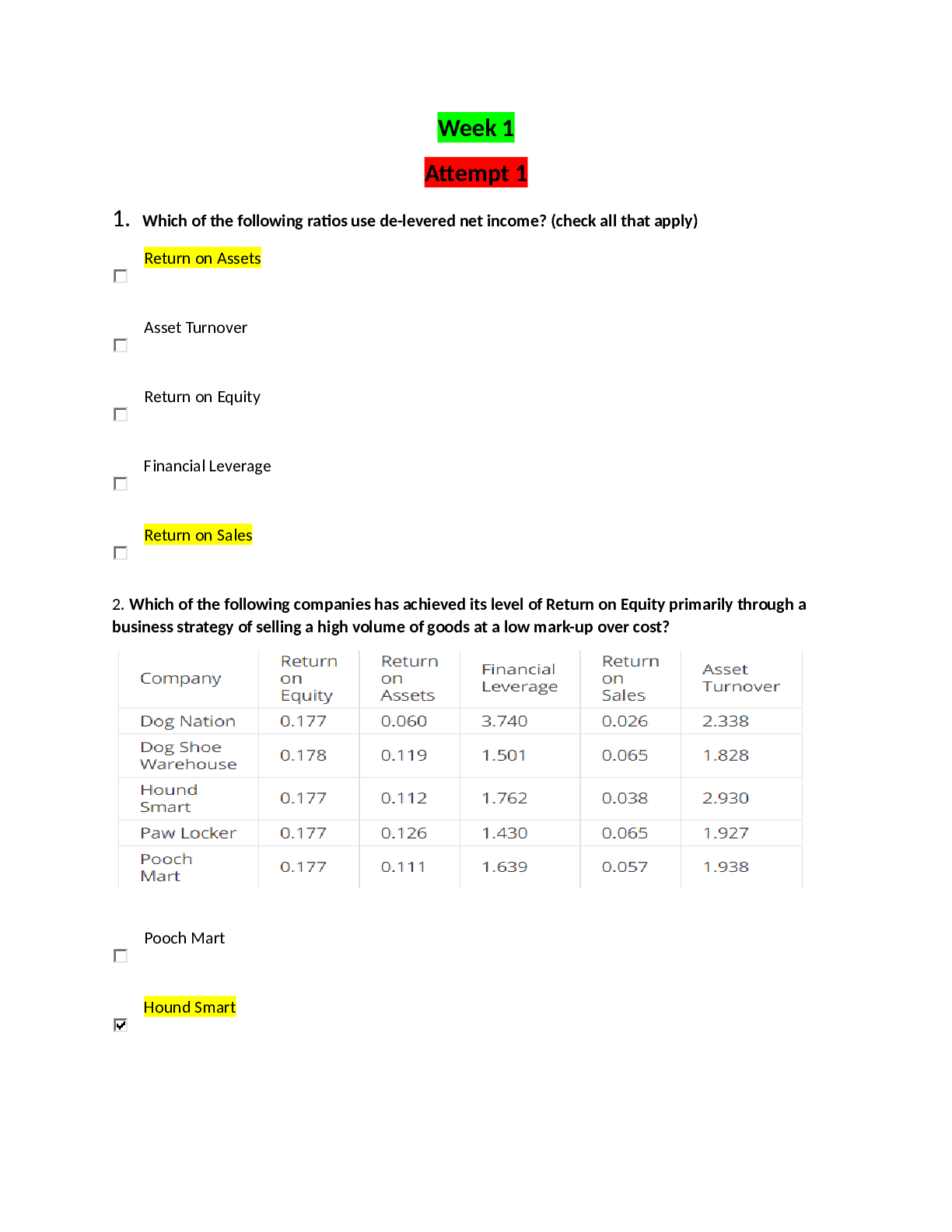
Introduction to Organic Chemistry. Questions and Answers.
Document Content and Description Below
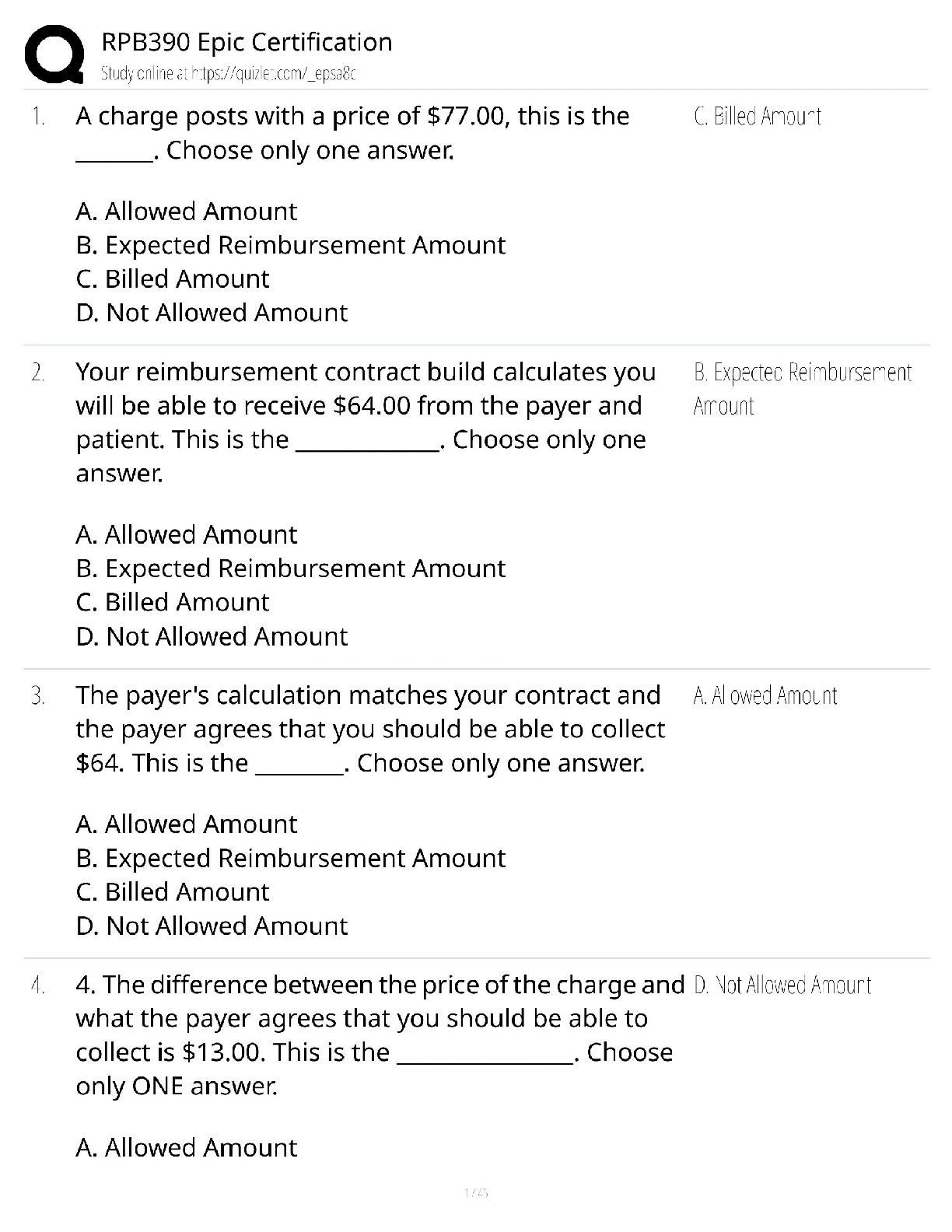
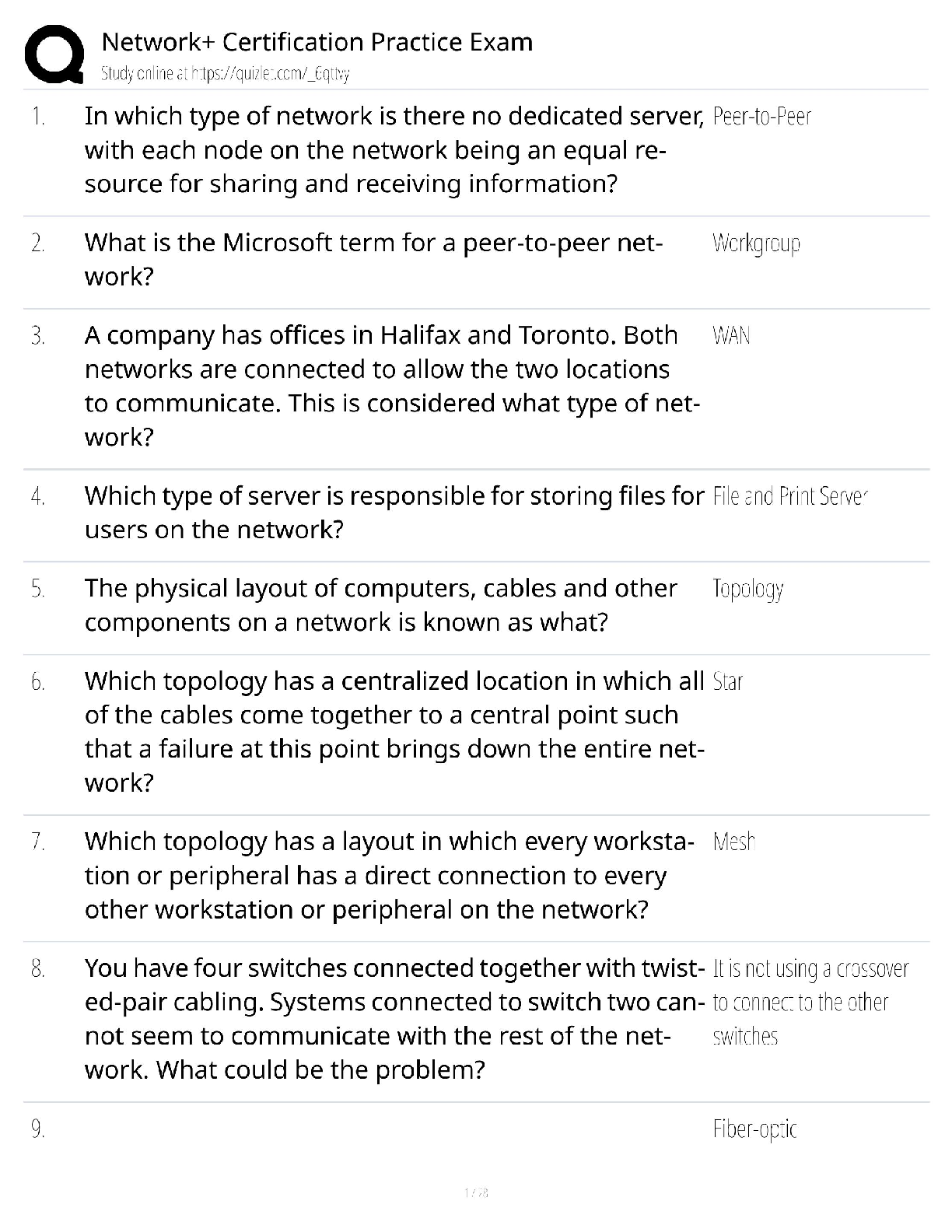
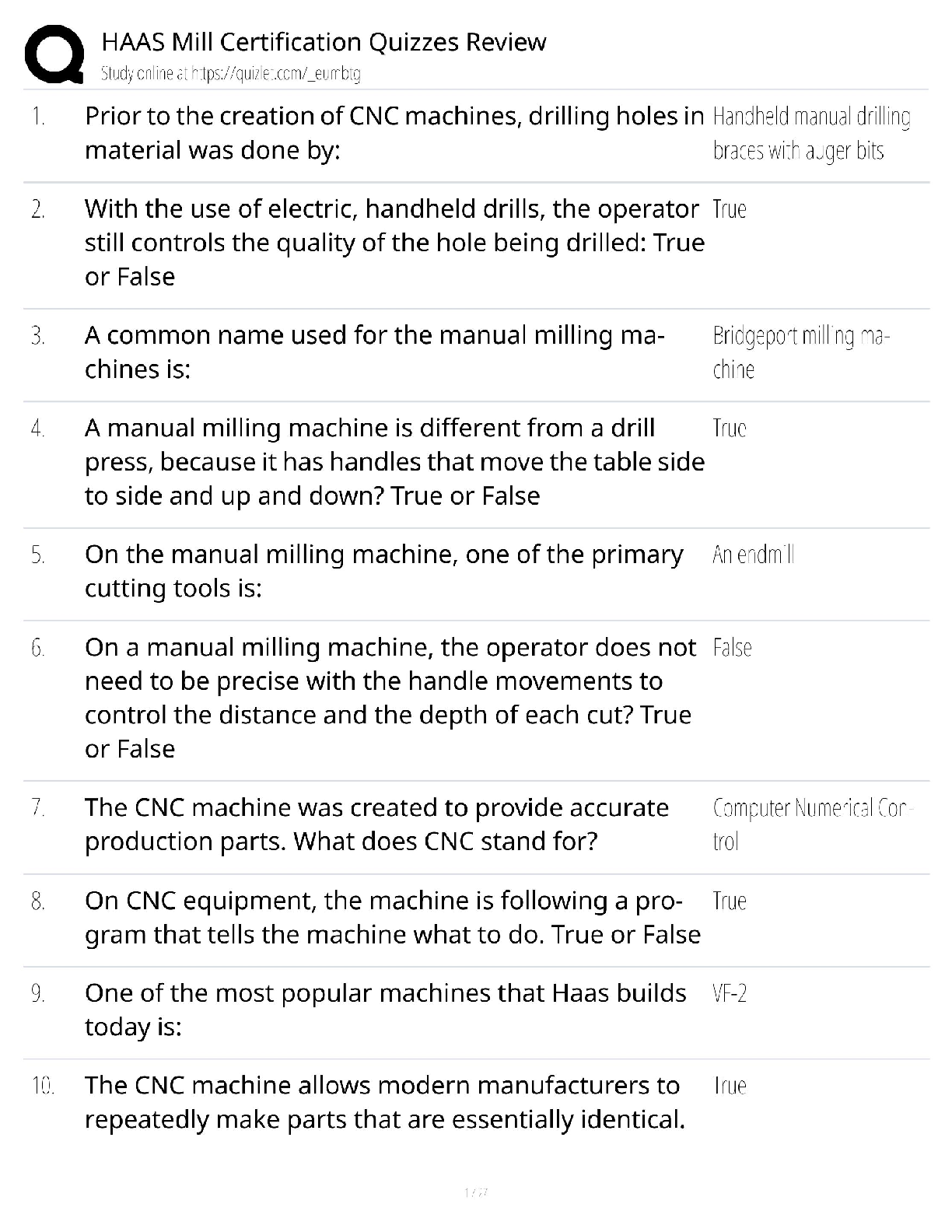
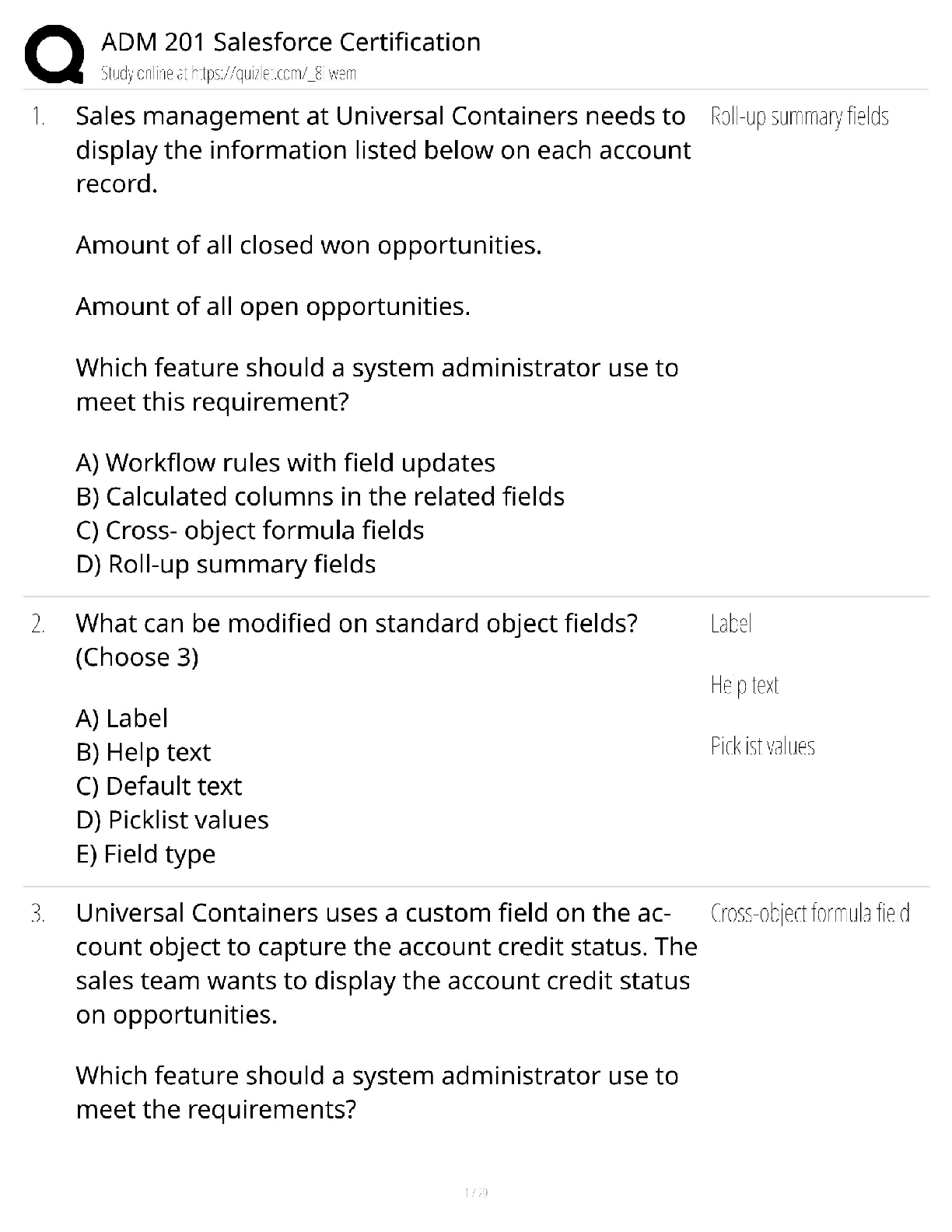
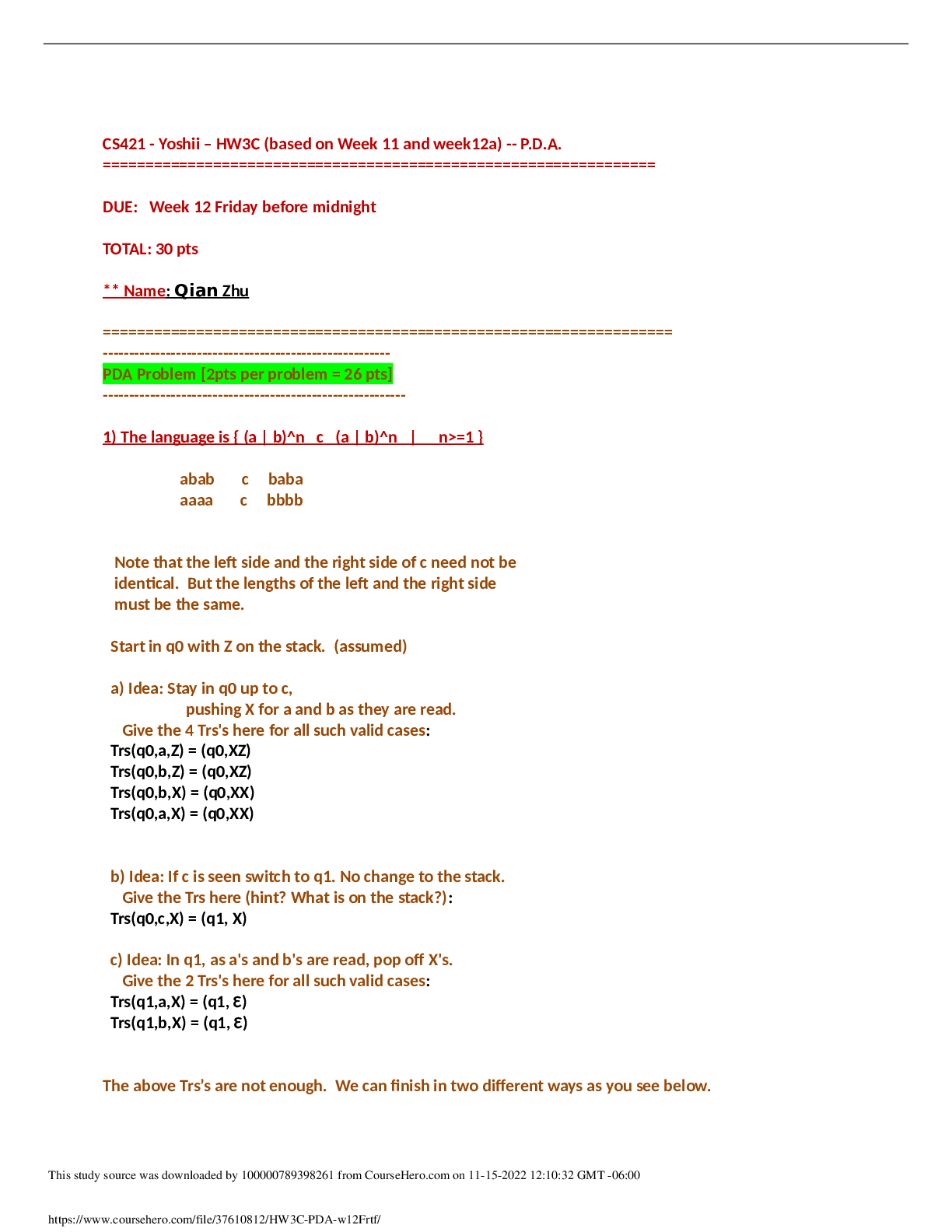
1. Chemical reactions occur as a result of: A) Attraction between opposite charges B) Nucleus–Nucleus interactions C) Motion of electrons D) Like atoms interacting E) Combining two chemicals ... 2. Credit for the first synthesis of an organic compound from an inorganic precursor is generally ascribed to: A) Berzelius B) Arrhenius C) Kekulé D) Wöhler E) Lewis 3. What was long thought to be the difference between inorganic and organic compounds? A) The number of atoms B) The synthesis of organic compounds required a vital force C) The molecular weight D) Inorganic compounds exhibited a strong nuclear force 4. Inorganic compounds were composed exclusively of transition metals Constitutional isomers may not differ in what aspects? A) Physical properties B) Atomic connectivity C) Molecular formula D) Name E) Constitution 5. What is the relationship between the following compounds? H O H H H H C C H H C C C H H H H C C C C H H H C H H A) They are isotopes B) They are constitutional isomers C) They are the same structure D) They are composed of different elements E) There is no relationship 6. What is the relationship between the following compounds? H H H H H H H C C O C H H C C C O H H H H H H H A) Resonance isomers B) Constitutional isomers C) Empirical isomers D) There is no relationship 7. Carbon generally forms four bonds and is considered: A) Tetravalent B) Divalent C) Trivalent D) Monovalent E) Qudravalent 8. Which of the following compounds are constitutional isomers of each other? H H H Br C C H C C C C H C Cl H H C H H Br C H C H C Cl C C C C C H H H H C C H C C H C C Br C H H Br H H C C C H H C C H C C Br C H H H H H I II A) I & II III Cl IV Cl B) III & IV C) I, II & III D) II, III & IV E) All of these 9. Which of the following compounds are constitutional isomers of each other? Cl H H Cl H Cl C C Cl H H C C Cl H H Cl H H H Cl I II III IV A) I & II B) III & IV C) II & III D) I & IV E) All of these Difficulty Level: Medium 10. Which of the following compounds are constitutional isomers of each other? H H H H H C N C O C C H H C H H N H C O H H H H C C C N C H H O H C C H H H H H C C H H H H H C N H H C I A) I & II H II III IV H H B) III & IV C) I, II & III D) I & IV E) All of these 11. Draw three constitutional isomers that have molecular formula C4H8BrCl Ans: H H H H H H H H H H H H H H H Cl H T 12. Draw three constitutional isomers that have molecular formula C4H8O. Ans: H H H H H O H H H C C C C O H H C C C C H H C C C O C H H H H H H H H H H H H There are additional correct answers 13. What force is not taken into account in the formation of a covalent bond? A) Repulsion between two positively charged nuclei B) Repulsion between electron clouds on individual atoms C) Force of attraction between positively charged nuclei and electrons D) Repulsion of electrons by neutrons E) All forces listed are involved in forming a covalent bond 14. What is the correct Lewis dot structure for S? S S S S S I A) I B) II C) III D) IV E) V II III IV V 15. What is the correct Lewis dot structure for C? C C C C C I A) I B) II C) III D) IV E) V II III IV V 16. What is the correct Lewis structure for PH3? H H P H H P H H P H P H H H P H H I H II A) I B) II C) III D) IV E) V P III IV P V 17. What is the correct Lewis structure for COCl2? C C O Cl C O Cl Cl C Cl Cl O Cl Cl O Cl Cl C Cl I A) I B) II C) III D) IV E) V 18. What is the correct Lewis structure for CH3CO2H? H H O H H C C O O H C H C O H H C O O C H H H I II H O H H III A) I B) II C) III D) IV E) V 19. Which of the following compounds has two lone pairs on the central atom? A) CO2 B) SCl2 C) NF3 D) CS2 E) SO3 20. What is the formal charge on oxygen in the following structure? H H C O H H H A) 2- B) 1- C) 2+ D) 1+ E) 0 Section: 1.4 Difficulty Level: Easy 21. What is the formal charge on nitrogen in the following structure? H H H H C N H H A) 2- B) 1- C) 2+ D) 1+ E) 0 Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 22. What is the formal charge on oxygen in the following structure? H O H C H C C H H H H A) 0 B) 1+ C) 2+ D) 1- E) 2- Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 23. What is the formal charge on oxygen in the following structure? H O H C C H H H H A) 2+ B) 2- C) 1+ D) 1- E) 0 Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 24. Which of the following structures have zero formal charge on carbon atom? H H H H H C H I A) I & III H C II H H C H C H III IV B) II & III C) III & IV D) I & IV E) II & IV Ans: C Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 25. Which of the following structures have 1- formal charge on sulfur atom? H H C S H H H C S H H S C H O H H O H C S H IV O H H O S O H O V A) I B) II I II III C) III D) IV E) V Ans: A Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 26. Which of the following structures have 1+ formal charge on sulfur atom? H H C S H H H C S H H S C H O H H O H C S H IV O H H O S O H O V A) I B) II I II III C) III D) IV E) V Ans: C Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 27. What are the formal charges on boron and fluorine in the following structure? F H F B N H F H A) B = 1+, N = 1+ B) B = 1+, N = 1- C) B = 1-, N = 1- D) B = 1-, N = 1+ E) B = 1-, N = 0 Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Easy 28. What are the formal charges on boron and oxygen in the following structure? H H F C H F B O F C H H H A) B = 1-, O = 1- B) B = 1-, O = 1+ C) B = 1+, O = 1+ D) B = 1+, O = 1- E) B = 1-, O = 0 Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Medium 29. Which of the following structures have 1+ formal charge on the central atom? H H H H . . . . . . . . H : Be : H H : B : H H : N : H H : N : H H : O : H . . . . . . . . I A) I B) II C) III H II III H IV V D) III & V E) IV & V Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Medium 30. Which of the following structures have formal charge on at least one atom? H H N F H O O H H C F H O B O H I II III H IV O H A) I B) II C) III D) IV E) None of these Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 31. Which of the following structures have 1- formal charge on nitrogen atom? H H C N H H C H H C N H H H C N H H H C N C H H O N H H H H H H H H H H V H A) I B) II C) III D) IV E) V I II III IV Topic: Identifying Formal Charges Section: 1.4 Difficulty Level: Medium 32. The bonding pattern of oxygen with a formal charge of –1 could be described as: A) One lone pair of electrons and three single bonds B) Two lone pairs of electrons and two single bonds C) Three lone pairs of electrons, and one single bond D) One lone pair of electrons, one single, and one double bond E) Zero lone pairs, and two single and one double bond Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 33. In ammonium ion, nitrogen has a valence of 4, and zero nonbonding electrons. What is the correct formal charge of nitrogen with 4 covalent bonds? A) 2- B) 2+ C) 1- D) 1+ E) 0 Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 34. What is the correct Lewis structure for nitric acid (HNO3) including the formal charges? O H O N O O N O H O O H N O O H O N A) I B) II C) III D) IV I II III IV E) None of these Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 35. What is the correct Lewis structure for hydrocyanic acid (HCN) including the formal charges, if any? H C N H C N H C N H C N I II III IV H C N V A) I B) II C) III D) IV E) V Ans: B Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Hard 36. What is the correct Lewis structure for SCN— including the formal charges, if any? A) I B) II C) III D) IV E) V Ans: A Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Hard 37. What is the correct Lewis structure for N2O including the formal charges, if any? N A) I B) II C) III D) IV E) V O N N O N N O N N O N N O I II III IV V Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Hard 38. What is the correct Lewis structure for hydrazoic acid (HN3) including the formal charges, if any? H N H N N N H N N N H N N N H N N N A) I B) II C) III D) IV E) V I II III IV V Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 39. Draw Lewis structure for NH2CN including formal charges, if any? Ans: H N C N H Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 40. Draw Lewis structure for –CH2CN including formal charges? Ans: H C C N H Topic: Identifying Formal Charges Section: 1.4, 1.3 Difficulty Level: Medium 41. Draw Lewis structure for ozone (O3,) including formal charges, if any? Ans: O O O Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 42. The electronegativity of elements on the periodic table tends to increase . A) From left to right, top to bottom B) From right to left, bottom to top C) From left to right, bottom to top D) From right to left, top to bottom E) F Ans: C Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 43. Which of the following is the least electronegative element? A) B B) C C) N D) O E) F Ans: A Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 44. Which of the following is the most electronegative element? A) B B) C C) N D) O E) H Ans: D Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 45. Which of the following is the least electronegative element? A) P B) N C) Mg D) Si E) K Ans: E Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 46. Which of the following is the most electronegative element? A) P B) N C) S D) O E) F Ans: E Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 47. What is the correct order of increasing electronegativity for Rb, F and O? A) Rb < F < O B) Rb < O < F C) O < F < Rb D) F < Rb < O E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 48. Which of the following series has the correct order of elements in increasing electronegativity? A) C < N < B < Br B) P < N < As < F C) Li < B < N < F D) Cl < Cs < C < Co E) Be < B < Ba < Br Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 49. The Cl—Cl bond is best described as _. A) Nonpolar covalent B) Polar covalent C) Ionic D) Coordinate covalent E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 50. The C—Cl bond is best described as . A) Nonpolar covalent B) Polar covalent C) Ionic D) Coordinate covalent E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 51. The bond between potassium and oxygen is best described as . A) Nonpolar covalent B) Polar covalent C) Ionic D) Coordinate covalent E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 52. The bond between carbon and hydrogen is best described as . A) Nonpolar covalent B) Polar covalent C) Ionic D) Coordinate covalent E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 53. Which of the following is the correct depiction of induction for a C—F bond? C F C F C F C F A) I B) II C) III D) IV I II III IV E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 54. Which of the following is the correct representation of dipole for P—Cl bond? + + + + Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Easy 55. Which of the following is the correct representation of partial charges at the indicated atoms? II I III C H C H H H A) I = +; II = +; III = + B) I = –; II = –; III = – C) I = +; II = +; III = – D) I = –; II = –; III = + E) I = +; II = –; III = + Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 56. Which atom has the most + in the following compound? Br H C N O Br A) N B) O C) Br D) H E) C Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 57. Which of the following statements best describes the C—Cl bond in the following compound? H H Cl C H C C H H H A) nonpolar; no dipole B) polar; + at carbon and – at chlorine C) polar; – at carbon and + at chlorine D) ionic E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 58. Which of the following compounds have both polar covalent and ionic bonds? A) NH4Br B) H2O2 C) HCN D) H2S E) None of these Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 59. For the following compound identify the polar covalent bonds and indicate the direction of dipole moment using + and -. O C H C H H Ans: H H O C H C H H H Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 60. For the following compound identify the polar covalent bonds and indicate the direction of dipole moment using + and -. O C H C N H H H Ans: Topic: Induction and Polar Covalent bond Section: 1.5, 1.3, 1.4 Difficulty Level: Medium 61. For NaSCH3, identify each bond as polar covalent, nonpolar covalent or ionic. Ans: polar covalent H Na S C H nonpolar covalent H ionic Topic: Induction and Polar Covalent bond Section: 1.5 Difficulty Level: Medium 62. For the following compound, identify each bond as polar covalent, nonpolar covalent or ionic and place a + on the most electropositive carbon. O C F H C Ans: H H polar covalent C + H C H H F polar covalent nonpolar covalent Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 63. Orbitals with the same energy are called . A) Quantum orbitals B) Atomic orbitals C) Antibonding orbitals D) Bonding orbitals E) Degenerate orbitals Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 64. What is the letter designation for the following atomic orbital? A) s B) p C) d D) f E) g Ans: B Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 65. What is the letter designation for the following atomic orbital? A) s B) p C) d D) f E) g Ans: C Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 66. In quantum mechanics a node (nodal surface or plane) is: A) location where is negative B) location where is positive C) location where 2 is positive D) location where 2 is negative E) location where is zero Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 67. Which of the following principle states that ―Each orbital can accommodate a maximum of two electrons with opposite spin‖? A) Aufbau principle B) Pauli exclusion principle C) Hund’s Rule D) Heizenberg Uncertainty principle E) Le Chatelier principle Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 68. Which of the following principle states ―When orbitals of equal energy are available, every orbital gets one electron before any gets two electrons‖? A) Aufbau principle B) Pauli exclusion principle C) Hund’s Rule D) Heizenberg Uncertainty principle E) Le Chatelier principle Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 69. Which of the following represents ground state electron configuration for phosphorous? A) 1s2 2s2 2p6 3s1 3p4 B) 1s2 2s2 2p6 3s2 3p4 C) 1s2 2s2 2p6 3s2 3p3 D) 1s2 2s2 2p6 3s2 3p2 E) 1s2 2s2 2p6 3s2 3p5 Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 70. The atomic number for nitrogen is 7. Which of the following represents ground state electron configuration for nitrogen? A) 1s2 2s1 2p4 B) 1s2 2p5 C) 2s2 2p5 D) 1s2 2s2 2p3 E) 1s2 2s2 3s3 Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 71. Which element has the electron configuration 1s2 2s2 2p6 3s2 3p5? A) oxygen B) fluorine C) sulfur D) chlorine E) bromine Ans: D Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Easy 72. Which element has the electron configuration 1s2 2s2 2p6 3s2 3p3? A) Cl B) S C) P D) Al E) N Ans: C Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 73. What is the electronic configuration for the nitride ion? A) 1s2 2s2 2p0 B) 1s2 2s2 2p2 C) 1s2 2s22p3 D) 1s2 2s22p4 E) 1s2 2s2 2p6 Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 74. What is the electronic configuration for the magnesium ion? A) 1s2 2s2 2p63s2 B) 1s2 2s2 2p6 C) 1s2 2s22p4 D) 1s2 2s22p63s1 E) 1s2 2s2 2p63s22p2 Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 75. What is the electronic configuration for the oxide ion? A) 1s2 2s2 2p6 B) 1s2 2s2 2p2 C) 1s2 2s22p4 D) 1s2 2s02p6 E) 1s2 2s2 2p63s22p2 Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 76. Which element has the following electronic configuration? 1s 2s 2p A) boron B) carbon C) silicon D) nitrogen E) fluorine Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 77. Which element has the following electronic configuration? 1s 2s 2p A) boron B) carbon C) silicon D) nitrogen E) fluorine Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 78. The following ground state electron configuration violates . 1s 2s 2p A) the Aufbau principle B) the Pauli Exclusion principle C) Hund’s Rule D) Heisenberg’s Uncertainty principle E) None of these Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 79. The following ground state electron configuration violates . 1s 2s 2p A) the Aufbau principle B) the Pauli Exclusion principle C) Hund’s Rule D) Heisenberg’s Uncertainty principle E) None of these Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 80. The following ground state electron configuration violates . 1s 2s 2p A) the Aufbau principle B) the Pauli Exclusion principle C) Hund’s Rule D) Heisenberg’s Uncertainty principle E) None of these Topic: Atomic Orbitals Section: 1.6 Difficulty Level: Medium 81. Ar, K+, and Cl– have equal numbers of electrons, and are considered isoelectronic. Provide the ground state electron configuration for them. Ans: 1s2 2s2 2p6 3s2 3p6 Topic: Valence Bond Theory Section: 1.7 Difficulty Level: Easy 82. Constructive interference of waves results in . A) a wave with smaller amplitude B) a wave with larger amplitude C) cancellation of both waves D) formation of a node E) Both C & D Topic: Valence Bond Theory Section: 1.7 Difficulty Level: Easy 83. Destructive interference of waves results in . A) a wave with smaller amplitude B) a wave with larger amplitude C) cancellation of both waves D) formation of a node E) Both C& D Topic: Valence Bond Theory Section: 1.7 Difficulty Level: Easy 84. All single bonds can be classified as: A) nonpolar covalent B) polar covalent C) ionic D) bonds E) bonds Ans: D Topic: Valence Bond Theory Section: 1.7 Difficulty Level: Easy 85. Which of the bonding type has circular symmetry with respect to the bond axis? A) bond B) bond C) bond D) covalent bond E) ionic bond Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 86. The difference between valence bond theory and molecular orbital (MO) theory is: A) valence bond theory requires the linear combination of atomic orbitals B) MO theory requires the linear combination of atomic orbitals C) valence bond theory considers only individual atomic orbitals D) Both A & B E) Both B & C Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 87. How many molecular orbitals are formed, when the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule? A) 1 B) 2 C) 3 D) 4 E) 5 Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 88. Which molecular orbitals are formed, when the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule? A) Two bonding molecular orbitals B) Only one bonding molecular orbital C) One bonding and one antibonding molecular orbital D) Two antibonding molecular orbitals E) Only one antibonding orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 89. How are electrons distributed in the molecular orbitals, when the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule? A) 2 electrons in the bonding molecular orbital B) 1 electron in the bonding molecular orbital, 1 electron in the non–bonding molecular orbital C) 1 electron in the bonding molecular orbital, 1 electron in the antibonding molecular orbital D) 2 electrons in the antibonding molecular orbital E) 2 electrons in the non–bonding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Easy 90. According to molecular orbital theory the highest energy molecular orbital that is occupied with an electron is referred to as: A) degenerate B) antibonding C) the LCAO D) the LUMO E) the HOMO Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Easy 91. According to molecular orbital theory the lowest energy molecular orbital that is unoccupied with an electron is referred to as: A) degenerate B) antibonding C) the LCAO D) the LUMO E) the HOMO Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Hard 92. Which of the following statement is incorrect, when the 1s atomic orbitals of two hydrogen atoms results in constructive interference? A) A sigma bonding molecular orbital is formed B) The bonding molecular orbital formed is lower in energy than the 1s atomic orbital C) The bonding molecular orbital formed has a node between the atoms D) The bonding molecular orbital formed has circular symmetry E) A maximum of two electrons may occupy the bonding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Hard 93. Which of the following molecules could not exist, according to the molecular orbital theory? A) He2 B) H2+ C) Li2 D) He2+ E) N2 Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 94. According to molecular orbital theory, the constructive interference of two atomic orbitals results in . onding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 95. According to molecular orbital theory, the destructive interference of two atomic orbitals results in . ntibonding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 96. Interaction of the following two atomic orbitals results in what kind of molecular orbital, in the orientation shown? + Ans: bonding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 97. Interaction of the following two atomic orbitals results in what kind of molecular orbital, in the orientation shown? Ans: bonding molecular orbital Topic: Molecular orbital Theory Section: 1.8 Difficulty Level: Medium 98. Interaction of the following two atomic orbitals results in what kind of molecular orbital, in the orientation shown? Ans: antibonding molecular orbital Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 99. What is the hybridization state of the oxygen atom in the following compound? H H C O H H A) sp B) sp2 C) sp3 D) sp3d E) s2p Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 100. What is the hybridization state of the nitrogen atom in the following compound? H H C N H H H A) sp B) sp2 C) sp3 D) sp3d E) s2p Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 101. What is the hybridization state of the boron atom in the following compound? F F B F A) sp B) sp2 C) sp3 D) sp3d E) s2p Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 102. What is the hybridization state of the carbon (I) atom in the following compound? H H C H A) sp B) sp2 C) sp3 I C N H H D) sp3d E) sp3d2 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 103. What is the hybridization state of the nitrogen atom in the following compound? H H C H A) sp B) sp2 C) sp3 D) sp4 E) s2p C N O H H Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 104. The lone pairs of electrons of the nitrogen atom are located in which orbitals? H H C H A) sp2 B) sp3 C) sp D) s E) p C N O H H Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 105. Which of the following structures have carbon with sp2 hybridization state? H H H H C H C H C H H C H I A) I & II II III H IV H B) III & IV C) I & III D) II & IV E) I & IV Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 106. Which of the indicated carbon atoms have sp2 hybridization state in the following compound? H O H C C C H H I II C H C H IV III A) I & II B) III & IV C) II & III D) I & III E) II & IV Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 107. What is the correct order of hybridization state for the numbered carbon atoms in the following compound? H H H C C C C H H H H H C C H H III I II A) I = sp3, II = sp2, III = sp B) I = sp2, II = sp, III = sp2 C) I = sp, II = sp2, III = sp3 D) I = sp, II = sp2, III = sp E) I = sp2, II = sp3, III = sp2 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 108. How many s—sp2 sigma bonds are in the following compound? H H H H C C C H C C H H H A) 2 B) 3 C) 4 D) 5 E) 6 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 109. The C1—C2 bond in the following compound results from the overlap of which orbitals? H O H C 1 C H C C 2 C H H H H H A) sp–sp2 B) sp–sp3 C) sp2–sp2 D) sp2–sp3 E) sp3–sp2 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 110. The bond that is part of C=C in the following compound results from the overlap of which orbitals? H O H C C H C C C H H H H H A) sp–sp2 B) sp–sp3 C) sp2–sp2 D) sp2–sp3 E) sp3–sp2 Ans: C Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 111. The C—C bond in ethyne (H—CC—H), results from the overlap of which orbitals? A) sp–sp B) sp–sp3 C) sp2–sp2 D) sp–s E) p–p Ans: A Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 112. How many bonds are present in the following compound? H O H C H A) one B) two C C H C C H H H H C) three D) four E) five Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 113. How many bonds are present in the following compound? H H H H H H H C C C C C C C C C C C H H H H H C H H A) two B) three C) four D) five E) six Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 114. How many bonds are present in the following compound? H H H H H H H C C C C C C C C C C C H H H H H C H H A) 20 B) 22 C) 24 D) 25 E) 27 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 115. The bond that is part of C=N in the following compound results from the overlap of which orbitals? H C N H C O H H H A) sp2–sp2 B) sp–sp C) sp2–sp3 D) sp3–sp3 E) sp3–sp2 Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 116. The bonds indicated by the arrow in the following compound results from the overlap of which orbitals? H H C C C C C H H H H A) sp2–sp2 B) sp3–sp3 C) p–p D) Both A & B E) Both A & C Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 117. Which orbitals are involved in the C—O, bond in acetone, shown below? O H C H C C A) C H H H H 2—O 2 sp sp B) C 3—O 3 sp sp C) Csp—Osp D) Cp—Op E) Csp—Op Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 118. Which of the following best describes the orbitals involved in the formation of C=O bond in acetone, shown below? O H C H C C H H H H A) Csp 2 – Osp 2 and Csp 2 Osp 2 B) Csp 2 – Osp 2 and Cp Op C) Csp 3 – Osp 2 and Cp Op D) Cp – Op and Csp 2 Osp 2 E) Csp – Osp and Cp Op Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 119. The C—H bond in the methyl cation, CH3+, results from the overlap of which orbitals? A) sp3–sp2 B) sp3–sp3 C) sp2–s D) sp3–p E) p–s Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 120. The lone pair of electrons in the methyl anion, CH3—, resides in which orbitals? A) s2 B) p C) sp D) sp3 E) sp2 Ans: D Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Hard 121. What is the hybridization state of the indicated atoms in the following compound? H O C N C H I II IV III A) I – sp ; II – sp2 ; III – sp3 ; IV – sp2 B) I – sp2 ; II – sp ; III – sp2 ; IV – sp3 C) I – sp3 ; II – sp2 ; III – sp ; IV – sp2 D) I – sp2 ; II – sp3 ; III – sp2 ; IV – sp E) I – sp2 ; II – sp2 ; III – sp2 ; IV – sp3 Ans: B Topic: Hybridized Atomic orbitals Section: 1.9, 1.3 Difficulty Level: Hard 122. The carbon and oxygen atoms in carbon monoxide are connected by which type of bond(s)? A) A sigma () bond B) Two sigma () bonds C) A pi () bond D) Two pi () bonds E) Both A and D Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 123. The N—H bond in the following compound is a and is formed from the . H H C N H H H A) σ bond; sp2 – s orbital overlap B) σ bond; sp3 – s orbital overlap C) π bond; sp3 – s orbital overlap D) π bond; sp2 – p orbital overlap E) π bond; p – p orbital overlap Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 124. Which is the shortest bond in the following compound? H H C C C C C H A) I B) II C) III D) IV H H I II H I IV E) I & III have the same length Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Easy 125. Which of the following compounds contains the shortest carbon–carbon bond? H H H H H H C C H H C C H H C C C H H C C H H H H H I II A) I B) II C) III D) IV H H H III IV E) All of these Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 126. Compare the bond length and strength for the following compounds. H C C H H C C H I H H II A) The shortest and strongest bond is found in compound I B) The shortest and strongest bond is found in compound II C) The shortest and weakest bond is found in compound I D) The shortest and weakest bond is found in compound II E) The bonds are of identical length and strength Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 127. Which is the longest C—C bond in the following compound? H H H C C C C C C H H H H H I A) I B) II C) III D) I & III E) All of these II III Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 128. Rank the indicated C—C bonds in increasing order of bond length. H H H C C C C C C H H H H H I A) I<II<III B) II<III<I C) III<I<II D) II<I<III E) I<III<II II III Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Medium 129. For the hydrogen halides, which is the correct sequence for a) the molecule with the weakest bond, b) the molecule with the shortest bond, and c) the molecule with the most polar bond? A) HF HI HBr B) HI HBr HCl C) HBr HI HF D) HI HF HF E) HCl HBr HI Topic: Hybridized Atomic orbitals Section: 1.9 Difficulty Level: Hard 130. Rank the indicated bonds in decreasing order of bond length. H H H H H C C C C C C C N C O H H H I A) I>II>III>IV B) II>III>IV>I C) IV>III>II>I D) III>IV>I>II E) II>I>III>IV H H H H H II III IV Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 131. The molecular geometry of carbon tetrachloride, CCl4, is best described as: A) tetrahedral B) trigonal planar C) trigonal pyramidal D) square planar E) linear Ans: A Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 132. Which of the following compounds have trigonal planar molecular geometry? F B F I F A) I B) II C) III D) IV H O H II H H C H III H F N F IV F E) I & IV Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 133. Which of the following compounds have trigonal pyramidal molecular geometry? H F B F H O H H C H F N F I F II A) I B) II C) III D) IV III H IV F E) I & IV Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 134. Which of the following compounds have bent molecular geometry? H F B F H O H H C H F N F I F II A) I B) II III H IV F C) III D) IV E) I & IV Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 135. Which of the following compounds have trigonal planar molecular geometry? H C H H O H F B F H O H H C H I H II H III F IV V H A) I, II & III B) II & III C) III & V D) V only E) All of these Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 136. Which of the following compounds have tetrahedral electron geometry? H C H H O H F B F H O H H N H I H II H III F IV V H A) I, II & III B) I, II, IV & V C) III & IV & V D) IV & V E) All of these Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 137. Which of the following compounds have trigonal planar electron geometry? H C H O S O F B F H O H H N H I H II III F IV V H A) I, II & III B) I, II, IV & V C) III & IV & V D) II &III E) All of these Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 138. What is the molecular geometry at the central atom in CH2Br2? A) trigonal planar B) trigonal pyramidal C) sqaure planar D) tetrahedral E) None of these Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 139. What is the molecular geometry at the central atom in SOCl2? A) trigonal planar B) trigonal pyramidal C) sqaure planar D) tetrahedral E) bent Ans: B Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 140. What is the molecular geometry at the nitrogen atom in the following compound? H C N H C O H H H A) trigonal planar B) trigonal pyramidal C) linear D) tetrahedral E) bent Ans: E Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 141. Which compound does not have a linear molecular geometry? A) CO2 B) H2O C) HCl D) HCN E) C2H2 Ans: B Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 142. What is the approximate bond angle around the indicated carbon atom? H H H O C C H H C C H H H H A) 600 B) 900 C) 109.50 D) 1200 E) 1800 Ans: D Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 143. What is the approximate bond angle around the indicated carbon atom? H H A) 600 B) 900 C C O H C) 109.50 D) 1200 E) 1800 Ans: E Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Easy 144. What is the approximate bond angle around the nitrogen atom? H N H C C C C H C H H A) 900 B) 109.50 C) 1200 D) 1800 E) 1000 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 145. What is the approximate bond angle around sulfur atom in SOCl2? A) 900 B) 109.50 C) 1050 D) 1200 E) 1800 Ans: B Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 146. What is the hybridization state and approximate bond angle around carbon atom in HCN? A) sp2, 1200 B) sp, 1800 C) sp3, 109.50 D) sp3, 1200 E) sp, 1200 Ans: B Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 147. What is the hybridization state and approximate bond angle around nitrogen in the following compound? H H H H C C H N H H C H H A) sp2, bond angle greater than 109.5° B) sp2, bond angle less than 109.5° C) sp3, bond angle greater than 109.5° D) sp3, bond angle less than 109.50 E) sp2, bond angle exactly 109.50 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 148. What is the hybridization state and approximate bond angle around sulfur in the following compound? H S H C C H H H H A) sp2, bond angle greater than 109.5° B) sp2, bond angle less than 109.5° C) sp3, bond angle greater than 109.5° D) sp3, bond angle less than 109.50 E) sp2, bond angle exactly 109.50 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 149. What is the hybridization state and molecular geometry around sulfur atom in SOCl2? A) sp2, tetrahedral B) sp2, trigonal planar C) sp3, tetrahedral D) sp3, trigonal pyramidal E) sp2, trigonal pyramidal Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 150. What is the hybridization state and molecular geometry around carbon atom in COCl2? A) sp2, tetrahedral B) sp2, trigonal planar C) sp3, tetrahedral D) sp3, trigonal pyramidal E) sp2, trigonal pyramidal Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 151. What are the hybridization state, molecular geometry and approximate bond angle for methyl cation, CH3+? A) sp2, tetrahedral, 1090 B) sp2, trigonal planar, 1200 C) sp3, tetrahedral, 109.50 D) sp3, trigonal pyramidal, 1200 E) sp2, trigonal pyramidal, 1800 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Medium 152. What are the hybridization state, molecular geometry and approximate bond angle for methyl anion, CH3—? A) sp2, tetrahedral, 1090 B) sp2, trigonal planar, 1200 C) sp3, tetrahedral, 109.50 D) sp3, trigonal pyramidal, 109.50 E) sp2, trigonal pyramidal, 1800 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 153. Draw Lewis structure for SOCl2 and predict the hybridization state, molecular geometry and approximate bond angle around the central atom. Ans: O Cl S Cl S: sp3, tetrahedral, 109.50 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 154. Draw Lewis structure for COCl2 and predict the hybridization state, molecular geometry and approximate bond angle around the central atom. Ans: O Cl C Cl C: sp2, trigonal planar, 1200 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 155. Following is the structure for Propranolol, and antihypertensive drug. What are the hybridization state, molecular geometry and approximate bond angle at the indicated nitrogen and oxygen atom in Propranolol? H H H H H H H CH3 C C C N H H H CH3 Ans: N: sp3, trigonal pyramidal, 109.50 O: sp2, bent, 109.50 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 156. What are the hybridization state, molecular geometry and approximate bond angle at each nitrogen atom in the following compound? O H3C C H C O H CH3 N I H C C C C H II H N C H C H H H Ans: N(I): sp2, bent, 1200 N(II): sp2, trigonal planar, 1200 Topic: VSEPR Theory: Predicting Geometry Section: 1.10 Difficulty Level: Hard 157. Tryptophan is an essential amino acid important in the synthesis of neurotransmitter serotonin in the body. What are the hybridization state, molecular geometry and approximate bond angle at the indicated carbon and nitrogen atoms? I H C O H H C C N H H C C H H C C IV C H C C H C N H H (I): sp2, trigonal planar, 1200 C(II): sp3, tetrahedral, 109.50 C(III): sp2, trigonal planar, 1200 N(IV): sp3, trigonal pyramidal, 109.50 Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Easy 158. Which of the following covalent bonds has the largest dipole moment? A) C—C B) C—H C) C—O D) N—H E) H—F Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Easy 159. Which of the following compounds has no dipole moment? A) CH4 B) NH3 C) HF D) HCl E) HBr Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Easy 160. Which of the following compounds has polar covalent bonds? A) NH3 B) Na2O C) H2 D) KF E) Both A & C Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 161. Which of the following compounds have net dipole moment? A) CBr4 B) CO2 C) CH4 D) H2O E) C2H4 Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 162. Which of the following compounds does not have dipole moment? A) HCl B) NCl3 C) CO D) BF3 E) All have dipole moment Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 163. Which of the following compounds has net dipole moment of zero? H H C C C C H H H C C C C H Cl C C H H F F F H IV V A) I B) II C) III D) IV E) V I II III Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 164. Which of the following compounds have a dipole moment? H O C O N C O H N C S C O A) II B) III I II III IV C) II & III D) I, II & III E) II, III & IV Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 165. Rank the following compounds in order of decreasing (highest to lowest) dipole moment. H H C C C C H F I II H H C C F III A) I>II>III B) II>III>I C) I>III>II D) III>II>I E) II>I>III Ans: D Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Hard 166. Rank the following compounds in order of decreasing (highest to lowest) dipole moment. O O O H H H H H C C C C H H C C H C C O C C H H H H H H O H H I II III A) I>II>III B) II>III>I C) I>III>II D) III>I>II E) II>I>III Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 167. Which of the following compounds have a dipole moment? Indicate the direction of dipole moment. H H Cl C C C C H H I II ompound I has no dipole moment. Compound II has dipole moment. Cl H II Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 168. Which of the following compounds have a dipole moment? Indicate the direction of dipole moment. Br Br H C H C C C H C H F H C H C C C H C H Br I II ompound II has no dipole moment. Compound I has a dipole moment. Br Br H H C H C C C C H H C H Br I II Topic: Dipole Moments and Molecular polarity Section: 1.11 Difficulty Level: Medium 169. BF3 has a no dipole moment. a) Draw the Lewis structure for BF3, showing all nonbonding electrons. b) Indicate the polarity of every atom in the structure using δ+ and δ– notation, and c) explain why the molecule has no net dipole. Ans: F B F F The trigonal planar geometry of BF3 results in the cancellation of individual bond dipoles, producing a net molecular dipole of zero. Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 170. Which of the intermolecular forces listed below is generally considered the strongest? A) London dispersion forces B) Fleeting dipole-dipole interactions C) Dipole-dipole interactions D) Hydrogen bonding E) The Vital force Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 171. Which intermolecular force is generally considered the weakest? A) Ion-dipole interactions B) London dispersion forces C) Dipole-dipole interactions D) Hydrogen bonding E) Covalent bonding Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 172. Which intermolecular force is primarily responsible for base pairing, and stability, of the double helix in DNA? A) Ion-dipole interactions B) London dispersion forces C) Dipole-dipole interactions D) Hydrogen bonding E) Covalent bonding Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 173. What is the strongest intermolecular force present in the following compound? H H H H H C C C C O H H H H H A) Ion-dipole interactions B) London dispersion forces C) Dipole-dipole interactions D) Hydrogen bonding E) Covalent bonding Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 174. What is the strongest intermolecular force present in the following compound? H H H H H C C C C S H H H H H A) Ion-dipole interactions B) London dispersion forces C) Dipole-dipole interactions D) Hydrogen bonding E) Covalent bonding Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Medium 175. Which of the following statements best explains the observation that HF has the highest boiling point of all the hydrogen halides? A) The fluorine in HF is the smallest atom for all of the halogens B) Fluorine is the most electronegative of the atoms C) HF can participate in hydrogen bonding D) HF is very reactive and can react and dissolve glass E) HF is a weak acid, and doesn’t completely dissociate Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Medium 176. Which of the following compounds have the greatest fleeting dipole interactions between like molecules? H H H H H H H H C H H H H H H H C H H H H H H H H C C C C H H C C C C C H H C C C H H C C C C C C H H H H H H H H H H H C H H H H H H H A) I B) II C) III D) IV I II III H H H IV E) I, III & IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 177. Which of the following compounds have the highest boiling point? H H H H H H H H H H H H H H H H H C C C C C H H C C C C O H H C C O C C H H C C C Cl H H H H H H H H H H H H H H H H A) I B) II C) III D) IV E) II & IV Ans: B I II III IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 178. Which of the following compounds have the lowest boiling point? H H H H H H H H H H H H H C H H H H H H C C C C C H H C C C C O H H C C C C H H C C C Cl H H H H H H H H H H H H H H H H A) I B) II C) III D) IV E) II & IV Ans: C I II III IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 179. Which of the following compounds have the lowest boiling point? A) CH3Cl B) CH2Cl2 C) CH4 D) CHCl3 E) CCl4 Ans: C Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Easy 180. Which of the following compounds have the lowest boiling point? H H H H H H H H C H H H H H H H C H H H H H H H H C C C C H H C C C C C H H C C C H H C C C C C C H H H H H H H H H H H C H H H H H H H A) I B) II C) III D) IV E) I & III I II III H H H IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Medium 181. Rank the following compounds in decreasing (highest to lowest) order of boiling point? H H H H H H H H C H H H H H H H C H H H H H H H H C C C C H H C C C C C H H C C C H H C C C C C C H H H H H H H H H H H C H H H H H H H I II III H H H IV A) III>I>IV>II B) II>I>IV>III C) III>I>II>IV D) IV>II>I>III E) I>III>II>IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Hard 182. Rank the following compounds in decreasing (highest to lowest) order of boiling point? H H H H H H H H H H H H H C H H H H H H H C C C C C H H C C C C O H H C C C C H H C C C C N H H H H H H H H H H H H H H H H H H H I II III IV A) III>I>IV>II B) II>IV>I>III C) III>I>II>IV D) IV>II>I>III E) I>III>II>IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Hard 183. Rank the following compounds in decreasing (highest to lowest) order of boiling point? H H H H H H H H H H H H H H O H H C C C C O H H C C O C C H H O C C C O H H C C C C H H H H H H H H H H H H H H H H I II III IV A) III>I>IV>II B) II>IV>III>II C) III>I>II>IV D) IV>II>I>III E) I>III>II>IV Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Medium 184. Which of the following compounds have a higher boiling point? Explain why. H H H H H C H H H H H H H C C C C H H C C C C C H H H H H H H H H H I II ompound II has a higher boiling point than compound I. Compound II has larger surface area that allows for stronger fleeting dipole-dipole interactions between molecules. Topic: Intermolecular Forces and Physical Properties Section: 1.12 Difficulty Level: Medium 185. Which of the following compounds have a higher boiling point? Explain why. H H H H H H H H H C C C C O H H C C O C C H H H H H H H H H I II ompound I has a higher boiling point than compound II. Compound I has hydrogen bonding interactions between molecules. Compound II has dipole- dipole interactions that are weaker than hydrogen bonding interactions. Topic: Solubility Section: 1.13 Difficulty Level: Easy 186. Which of the following compounds is most soluble in water? H H H H H H H H H H H H H H H H H C C C C C H H C C C C O H H C C O C C H H C C C Cl H H H H H H H H H H H H H H H H A) I B) II C) III D) IV E) II & III I II III IV Topic: Solubility Section: 1.13 Difficulty Level: Easy 187. Which of the following compounds is most soluble in butane (CH3CH2CH2CH3)? H H H H H H H H H H H H H H H H H C C C C C H H C C C C O H H C C O C C H H C C C Cl H H H H H H H H H H H H H H H H A) I B) II C) III D) IV E) II & III I II III IV Topic: Solubility Section: 1.13 Difficulty Level: Medium 188. For soap to remove and dissolve oil in water, what molecular features are needed? A) One end of the molecule must be polar B) The compound must contain oxygen atoms C) One end of the molecule must be nonpolar D) Both A & C E) All A, B & C Topic: Solubility Section: 1.13 Difficulty Level: Medium 189. Amino acids are building blocks of proteins. Which statement best describes the physical properties of the following amino acid? O H H O C C N H H H A) high melting points and low solubility in water B) large dipole moments and no hydrogen bonding C) high melting points and large dipole moments D) low solubility in water and small dipole moments E) small dipole moments and are hydrophobic 190. Sugars, an example of which is shown below, tend to be very soluble in water. Explain why. H H OH H C HO H C C H C O HO C C H OH OH H (The lone pairs of electrons on the oxygen atoms are not shown for clarity) 191. Describe how soaps function as cleaning agents. [Show More]
Last updated: 3 years ago
Preview 1 out of 67 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$4.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 22, 2020
Number of pages
67
Written in
All
Additional information
This document has been written for:
Uploaded
Mar 22, 2020
Downloads
0
Views
240




.png)
.png)
.png)
.png)
.png)
.png)
.png)