Chemistry > QUESTIONS & ANSWERS > Questions and Answers > GIZMOS: Student Exploration: Trends in the Periodic table (All)
Questions and Answers > GIZMOS: Student Exploration: Trends in the Periodic table
Document Content and Description Below
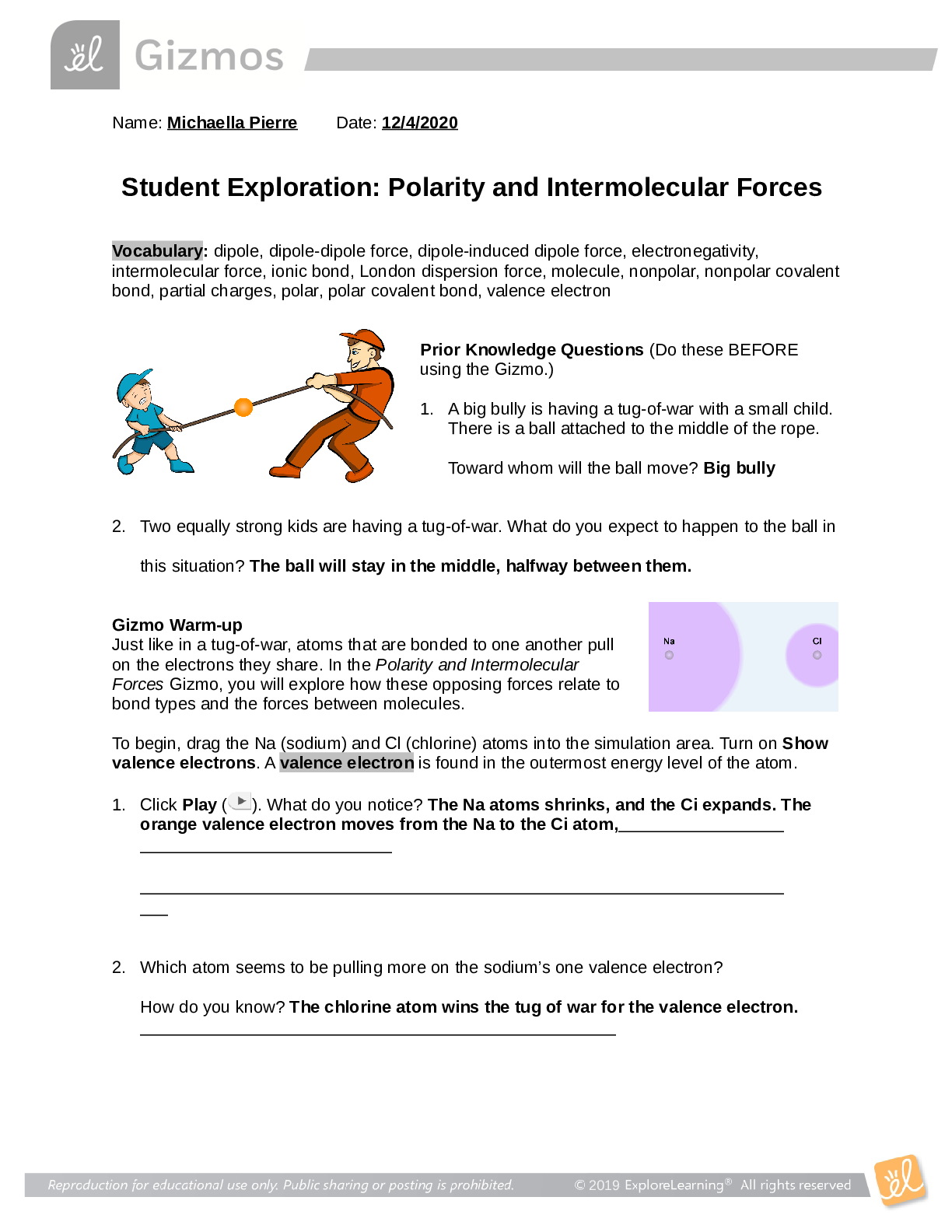
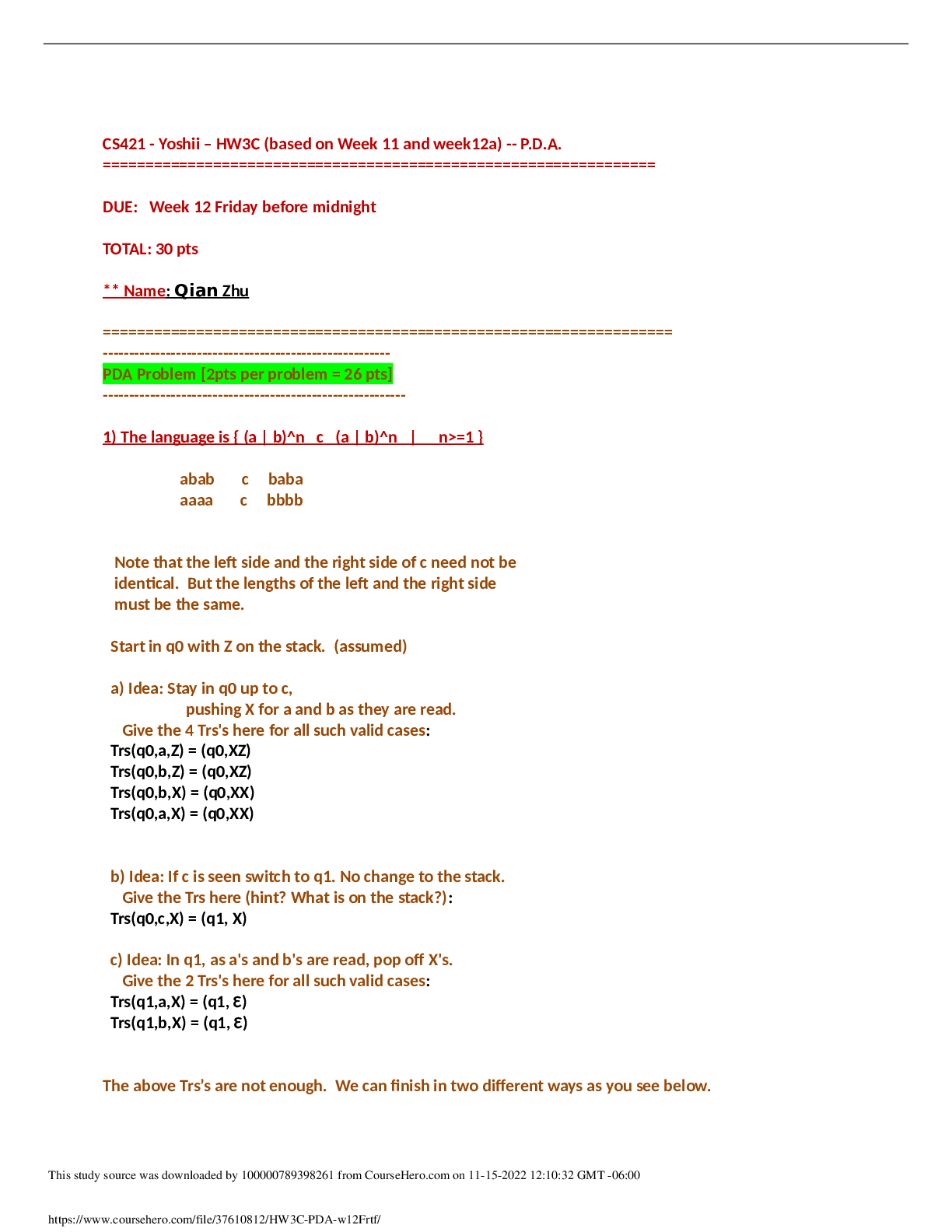
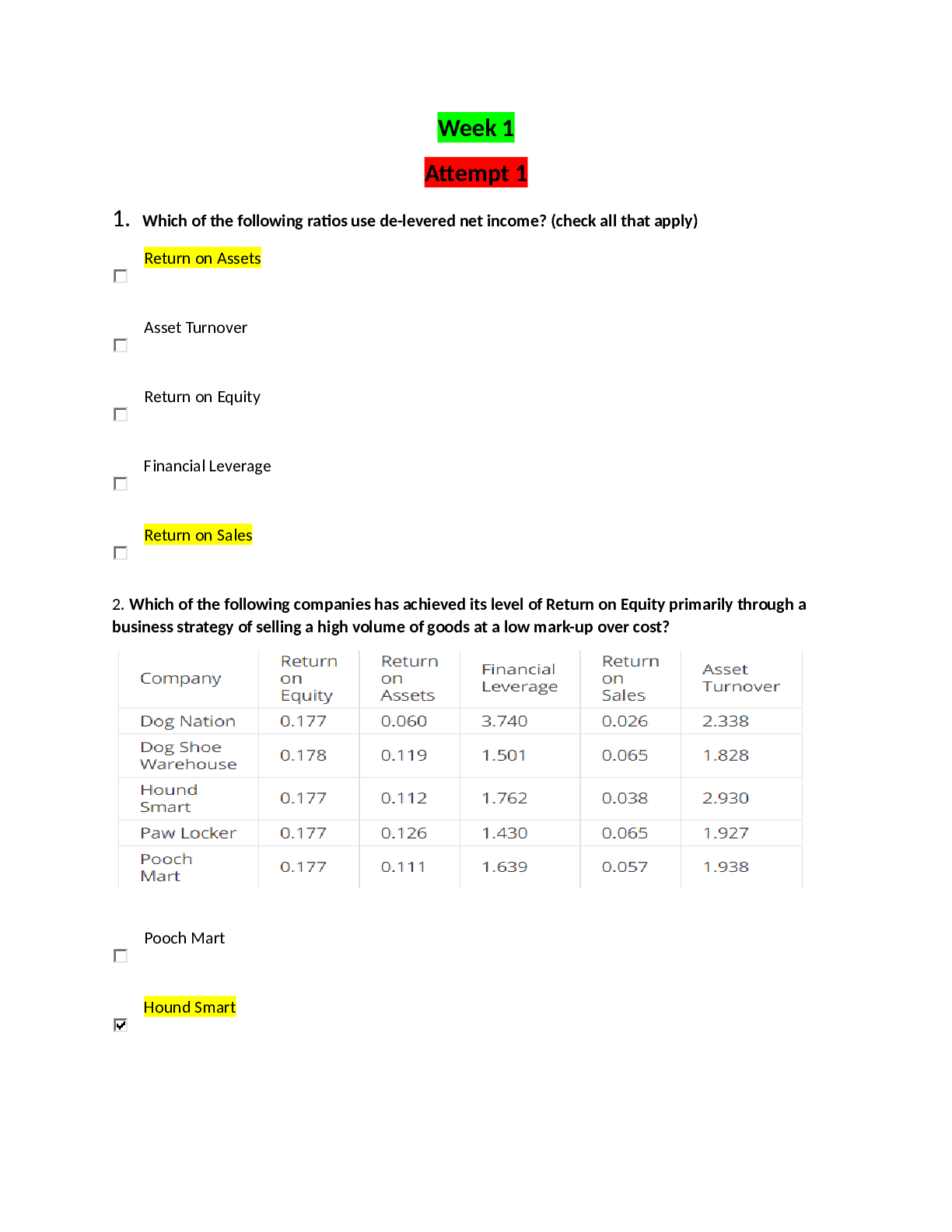
Trends in the Periodic table 1. Examine your graph of ionization energy (IE) vs. atomic number. A. Which elements are found at the main peaks of your graph (there should be three)?... What do these elements have in common? [2] B. Which elements are found at the main valleys of your graph (there should be three)? What do these elements have in common? [2] 2. Examine your graph of atomic radius vs. atomic number. They also give away electrons What do these elements A. Which elements are found at the main peaks of your graph? have in common? [2] B. Which elements are found at the main valleys of your graph? What do these elements have in common? [2] 3. How are the atomic radii and IE related (i.e. as the radius increases, what happens to the IE)? [2] 4. Generally, as you go from left to right across a period on the periodic table, what happens to atomic radius? What happens to IE? [2] 5. Generally, as you go down a group in the periodic table, what happens to atomic radius? What happens to IE? [2] 6. When Na forms an ion it loses its outer electron to become Na+. Draw a B-R diagram for BOTH Na and Na+. What element does Na+ resemble (with respect to electron arrangement)? In general, which groups’ electron configuration do the alkali metals resemble when they form ions? (I.e. lose an outer electron)? [4] 7. Why does the radius increase as you go down a group (hint: think of B-R diagrams)? Why would an increase in radius make it easier to lose an outer electron (i.e. give a lower ionization energy)? [3] 8. What happens to the number of protons in the nucleus as you go across a period? Use this to explain the trends in atomic radius and ionization energy across a period. [3] [Show More]
Last updated: 2 years ago
Preview 1 out of 3 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Feb 09, 2021
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Feb 09, 2021
Downloads
0
Views
117




.png)
.png)
.png)
.png)
.png)
.png)
.png)