BioChemistry > QUESTIONS & ANSWERS > Research Paper > Middle Georgia State UniversityBIOL 3104CH 15 & 16 Notes CHAPTER 15 INTRACELLULAR C (All)
Research Paper > Middle Georgia State UniversityBIOL 3104CH 15 & 16 Notes CHAPTER 15 INTRACELLULAR COMPARTMENTS AND TRANSPORT
Document Content and Description Below
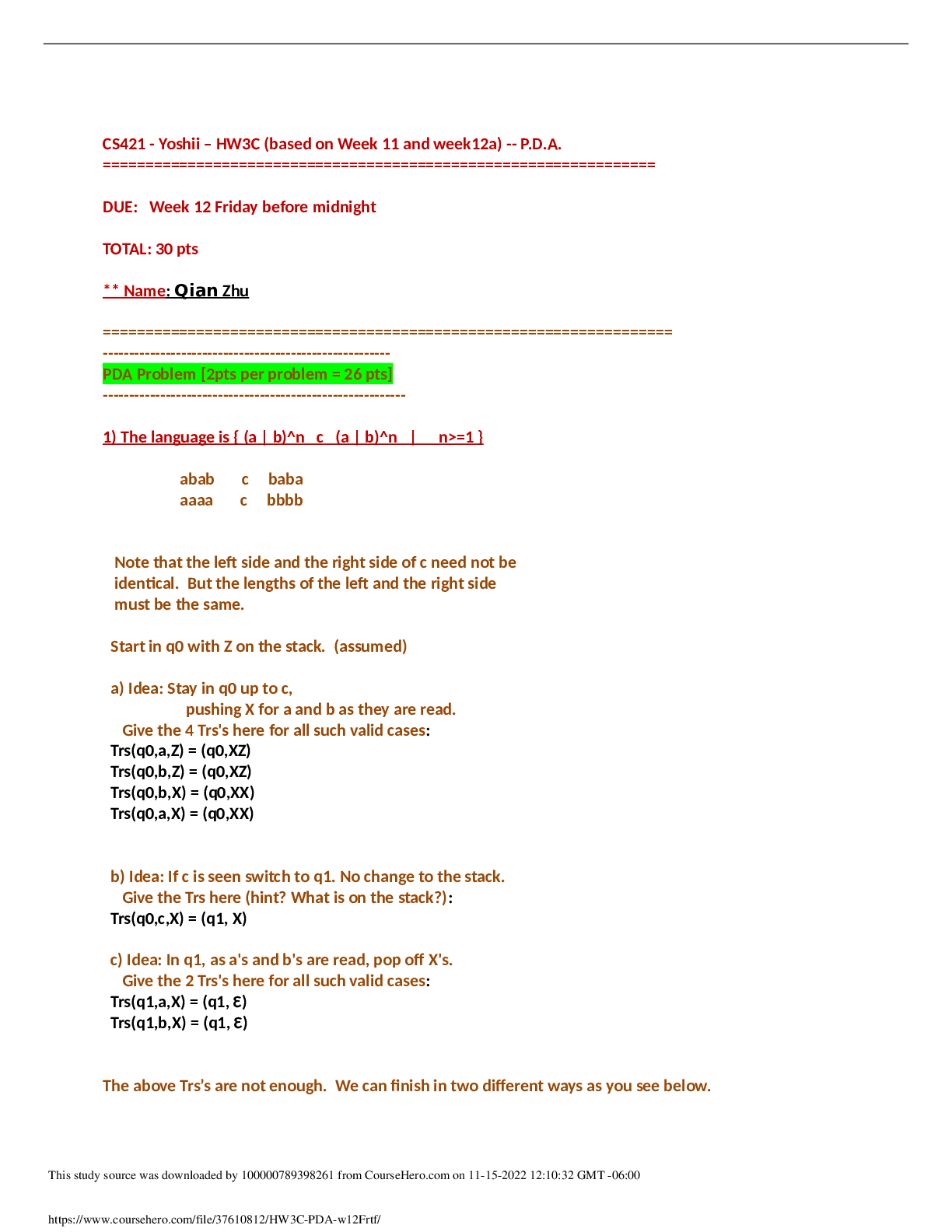
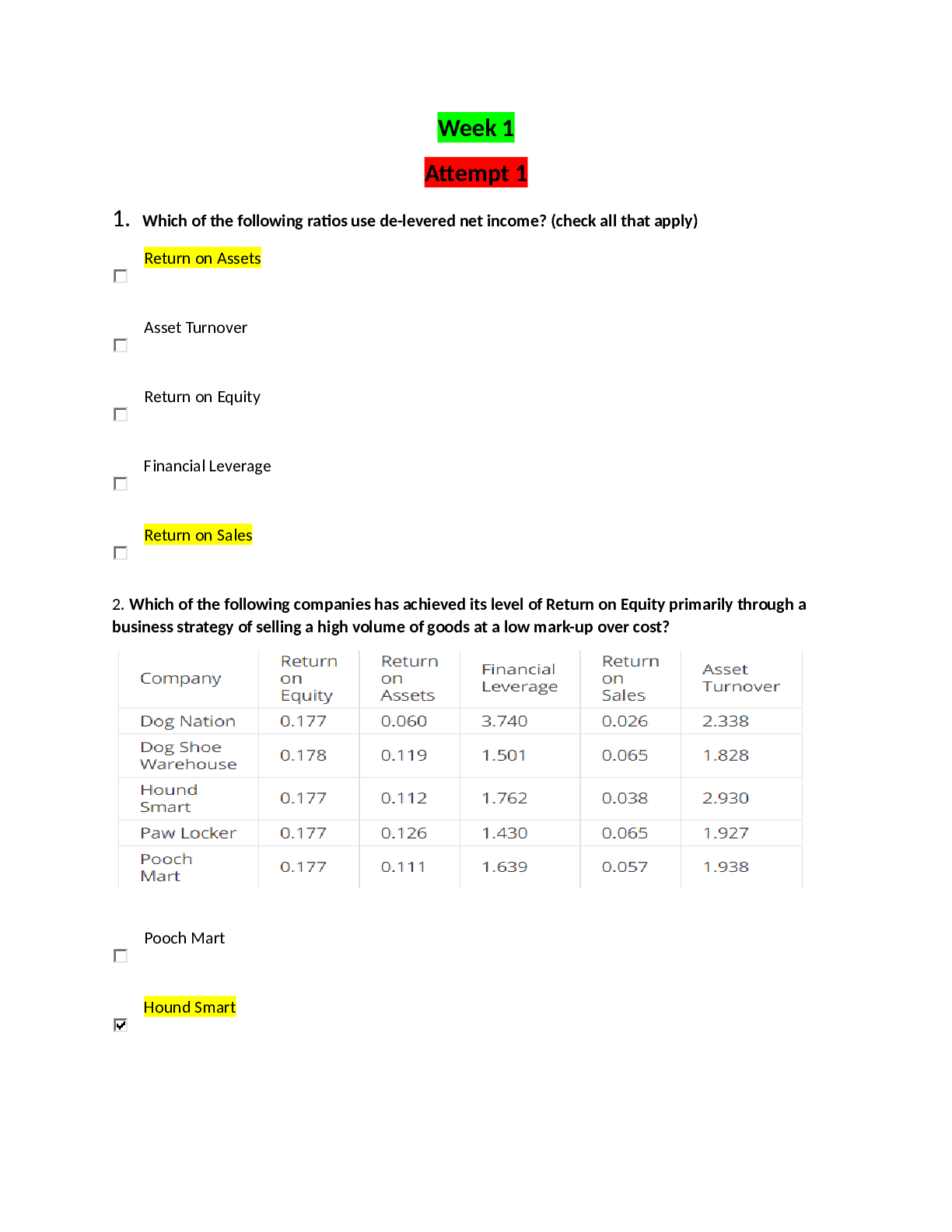
1 CHAPTER 15 INTRACELLULAR COMPARTMENTS AND TRANSPORT 1. list the 3 main ways newly made proteins are transported into membrane-enclosed organelles. Protein sorting: transport of protei... ns to proper location. o 3 types of transport §1. Through nuclear pores: selective, active transport large molecules and diffusion of small molecules. §2. Across membranes: protein translocators in membrane of organelle; unfolded proteins pass into lumen. §3. By vesicles: carried in membrane/lumen of transport vesicle between organelles or to plasma membrane. 2. What are signal sequences? (Table 15-3) Protein sorting depends on existence of particular amino acid sequence in protein. Signal sequences (sig seqs) identify proper, final location for protein and often cleaved off once protein is sorted. The signal sequence is almost like an “address on a letter” Experiments using recombinant DNA (altered gene sequence): signal sequence, alone, is sufficient to cause protein sorting. ER lumen protein remains in cytosol if signal sequence is removed and cytosolic protein sorts to ER if signal sequence is added. 3. Describe protein transport into the nucleus. (15-7) Proteins sorted to nucleus must pass through pores that perforate nuclear envelope. (15-8) Nuclear pores large, elaborate complexes of approximately 30 proteins. Wall surrounding waterfilled passage and meshwork of fibers (prevents passage of large molecules; allows small molecules through). Proteins like polymerases would have to be transported into the nucleus. 2 Nuclear proteins contain nuclear local signal sequences recognized by nuclear transport receptor in cytosol. Receptor assists entry into pore; interacts with tendrils from cytosolic side. Receptor/protein complex actively transported into nucleus. (15-9) Active transport of proteins in nucleus fueled by GTP hydrolysis. Nuclear transport receptor associates with RAN-GTP Guanine-Triphoshate (small GTP-ase) GTP is in a similar way used just like ATP is used; the difference is you need a molecule that breaks down GTP. [Watched nuclear import video] 4. Describe protein transport into mitochondria and chloroplasts. (15-11) Most proteins are transported from the cytosol. Signal sequence at N term recognized by transmembrane receptor. Protein translocators in outer and inner membrane align and move protein after receptor release. Protein unfolds as it crosses both membranes simultaneously. Signal sequence cleaved and protein refolds (with chaperones). Why are MOST proteins transported from the cytosol and not all? Double-membrane, have their own DNA 5. Describe protein transport into the ER. ER has luminal and membrane proteins; it also receives proteins to be transported to the Golgi, lysosomes, endosomes, and plasma membrane. Translocated during synthesis by ribosomes associated with ER. We can divide the proteins that have to go to the ER by two major groups: proteins that are going to become part of the ER itself—resident proteins (ones that live in the lumen or the ones that live in the membrane), translocated proteins Smooth and rough ER (place where proteins are being synthesized that either need to live permanently in the ER or needs to be transported. 3 (15-13) Free cytosolic ribosomes and RER ribosomes from common pool in cytosol. Synthesis location depends on protein sequence. If protein has N-term signal sequence for ER, ribosome attaches to ER. If not, stays in cytosol. ER protein threaded through translocation channel in ER membrane as translocation proceeds. Translating ribosome guided to ER membrane by signal recognition particle (SNP); binds N term sig sequence of elongation polypeptide (temp halts translation) SRP/cargo bind transmembrane receptor; ferried to translocation channel. SRP released and polypeptide/ribosome complex passed to channel where translocation recommences. (14) (15-15) luminal proteins/secreted proteins translocated through ER membrane. Signal peptidase cleaves signal sequence and releases mature polypeptide into lumen. Vesicles may bud from ER and carry proteins to other organelles. The signal sequence that identifies the ER membrane is hydrophobic, so to release the protein the enzyme peptidase cleaves. Transmembrane proteins (TPs) remain in the lipid bilayer. Single pass TPs: start signal sequence bound by SRP; binds receptor & carried to channel. Protein threaded through as translated until reaches hydrophobic stop transfer sequence. Threading stops; translation of cytosolic portion continues until completion. Signal sequence cleaved and TP released sideways into bilayer. (15-16) 4 Multipass Transmembrane proteins: more than 1 pari of start/stop signal sequences; portions of polypeptide cytosolic and others noncytosolic. (15-17) The signal sequence remains in the membrane and does not get cleaved off . WATCH ER TRANSLOCATION VIDEO 6. Distinguish between the 2 types of vesicular transport pathways. (15-18) a. Secretory pathwayàtransport vesicles from ER to Golgi to plasma membrane to release cargo into extracellular fluid or to place transmembrane proteins in the plasma membrane. b. Endocytic pathwayàThose that fused early with the endosomes (come from vesicles when the plasma membrane invaginates and pinches off & fuses with the Golgi) Plasma membrane invagination into transport vesicles, fusion with vesicles from Golgi to form endosomes and lysosomes for degradation of cargo. Notice that the membranes as they are forming into this vesicles are not naked. The cargo is onto the left of the invagination. On the other side we see a regular pattern of molecules all the way through the invagination. Scientists investigated to see the importance of these molecules. 5 Budding vesicles assisted by collection of proteins that coat cytosolic surface of vesicle. Proteins help shape membrane into bud and trap cargo to be transported. Coated vesicles lose coats to fused with other membrane. (15-19a) Best studied is clathrin. 1. Clathrin coats surround vesicles budding from Golgi and from plasma membrane (15-19b) 2. Clathrin proteins assemble into basket-like network on cytosolic surface of membrane forming pit that enlarges into bud. c. Receptor-mediated transport: Cargo receptors bind specific proteins (via signal sequence recognized) Adaptins bind loaded cargo receptors on one end and clathrins on other. Recruits cargo into coated pit. As the bud enlarges, dynamin encircles neck and squeezes to pinch off vesicle. Adaptins/clathrins released. Example: LDL proteins are taken in this way by cells. d. Fusion of vesicles with another membrane requires snares and tethers. (15-21) V-snare from vesicle; t-snare and tether are from the target membrane. The tether binds the RAB protein on surface of vesicle causing vesicle to dock with target membrane. Snares intertwine, pulling vesicle down to cause fusion of vesicle to the target membrane. 6 CHAPTER 16 Cell Communication 1. What are the basic steps of cell signaling? (16-2) Signal is produced by cell or environment. Signal received by transmembrane or intracellular receptor of target cell. Signal transduction: extracellular signal converted into intracellular signal to produce response. o What type of cell signaling did we talk about that the signal was not a molecule? The bending of the stereocilia in the ear. 2. Define the different types of signaling mechanisms and cite an example of each. (16-3) Cell to Cell signaling: 4 different types (endocrine, paracrine, neuronal, contact-dependent) o ENDOCRINE: circulating hormone (bloodstream or sap) broadcast to all cells. Ex: insulin/blood sugar o PARACRINE: local mediator released into extracellular fluid and then diffuses to nearby cells. o Ex: PDGF (Platelet derived growth factor)/wound healing o NEURONAL: Neurotransmitter is released into the synaptic cleft (<100nm) Diffuses to postsynaptic cell. Ex: ACH (Acetycholine)/ muscle contraction o CONTACT-DEPENDENT: membrane protein signal displayed to membrane receptor on adjacent cell. Ex. Delta (the name of the signal)àneuronal development §(16-4) Was discovered during experimentation with neurogenesis in fly embryos. One epithelial cell within a group expresses membrane protein Delta; This cell differentiates into a neuron. Delta binds to membrane receptor (Notch) on adjacent cells which inhibits their differentiation into neurons. 7 3. How do cells within multi-cellular organisms respond specifically to external signals? (16-5) Multicellular organisms: specific receptors and/or intracellular signals determine response to signal. Ex: ACH heart muscle inhibits contraction; salivary gland causes secretion. Same receptor but different intracellular proteins; different responses. Skeletal muscles have a different ACH receptor; causes contraction. Some cells no receptors for ACH and thus yield no response. (16-6) Most cells have a variety of receptors; different responses depending on availability of signals. Ex: same cell may survive, grow & divide, differentiate, or die (apoptosis); depends on presence/absence or particular signals. For example when you are developing, the skin cells between your digits (fingers) receive no signal and die by apoptosisàtherefore no webbing. 4. Distinguish between slow and fast responses to signals. (16-7) Extracellular signals may cause slow or fast responses in target cells. Slow response: receptor activation alters gene expression, protein synthesis, and cytoplasmic machinery (min to hr) Fast response: receptor activation alters protein function and cytoplasmic machinery (sec to min) What are the benefits of slower responses? o How daughter cells inherit particular expression? Epigenetics Hydrophilic/very large hydrophobic signals must bind to cell surfaces receptors (or actively transported) to mediate intracellular response. Small hydrophobic signals pass through lipid bilayer & thus bind intracellular receptors to induce response (represents slow response). (16-8) NOVEMBER 30, 2011 Cortisol (hydrophobic, stress) diffuses through bilayer and binds intracellular nuclear receptors. Activated receptor/cortisol transported [Show More]
Last updated: 2 years ago
Preview 1 out of 10 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jun 01, 2021
Number of pages
10
Written in
Additional information
This document has been written for:
Uploaded
Jun 01, 2021
Downloads
0
Views
111




.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)

