Chemistry > QUESTIONS & ANSWERS > Azusa Pacific University - CHEM 115 Week 4-Quiz wAns. 100% Pass rate. (All)
Azusa Pacific University - CHEM 115 Week 4-Quiz wAns. 100% Pass rate.
Document Content and Description Below
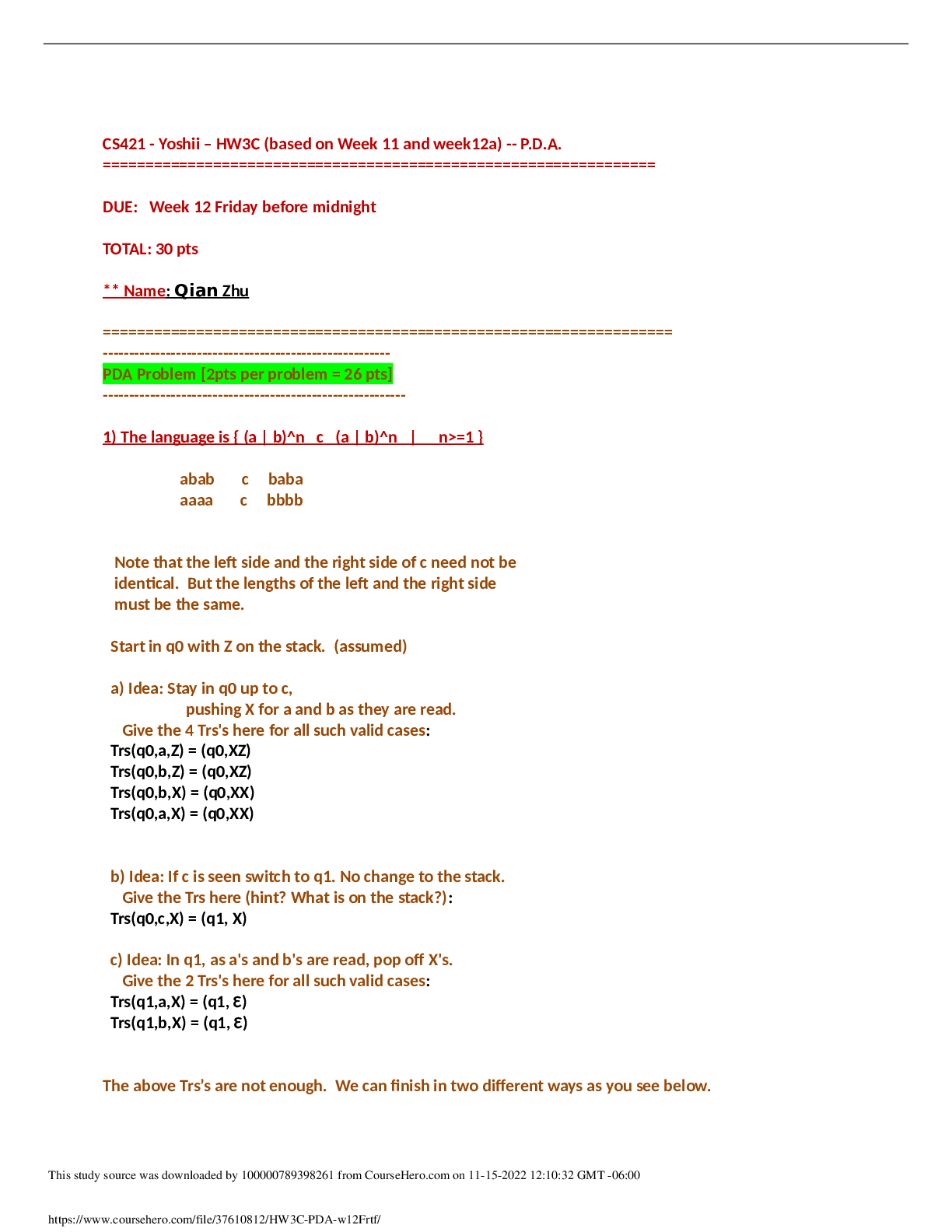
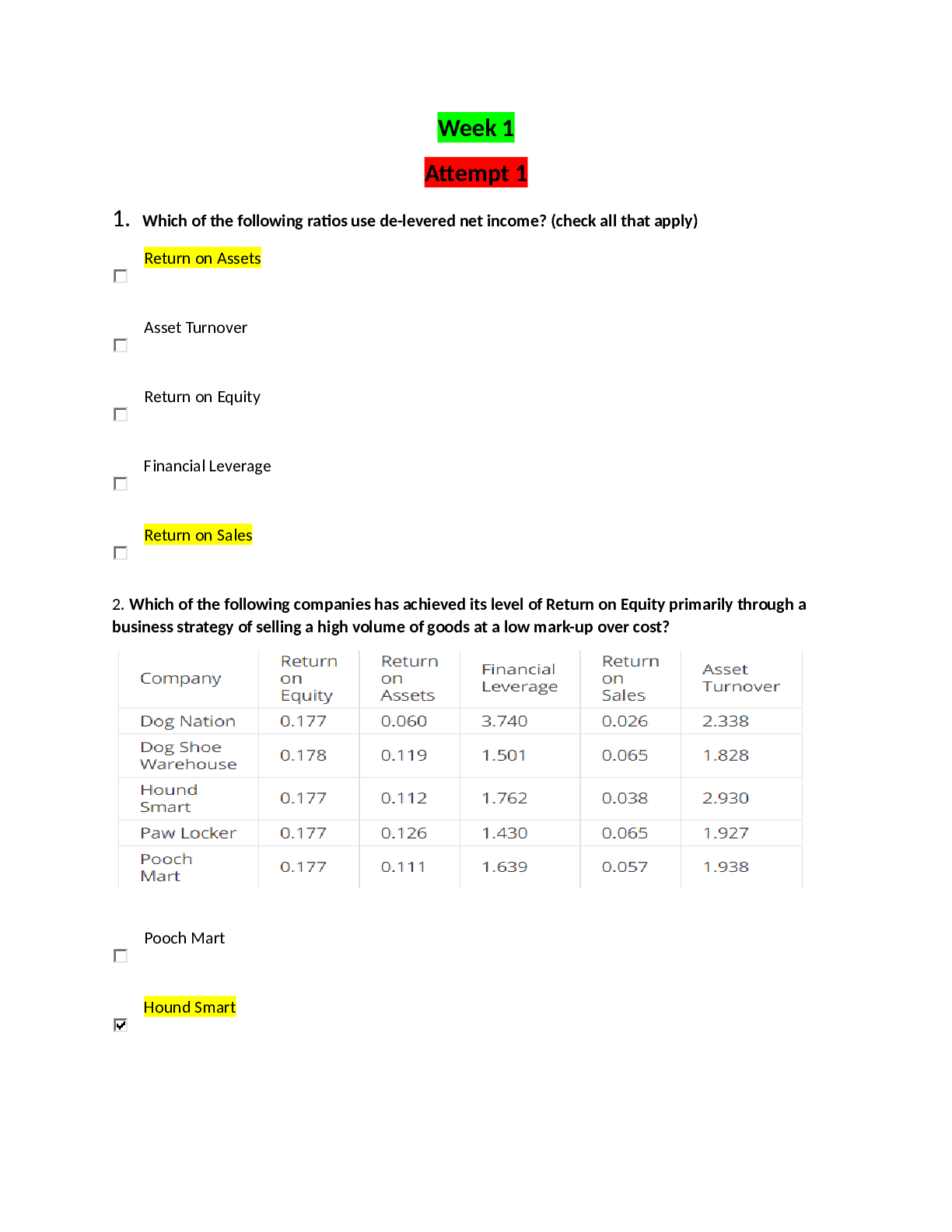
CHEM 115 Week 3 – Quiz ~ Ch. 7 & 8 Which of the following is the correct statement concerning the equation below? a. HSO4– is the conjugate base of SO4– b. HSO4– is the conjuga... te base of H3O+ c. H2O is the conjugate base of HSO4– d. SO42– is the conjugate base of HSO4– The carbonic acid/bicarbonate buffer is important in maintaining the proper pH of human blood. Diarrhea can lead to the loss of HCO3-. According to LeChatelier’s principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH. a. left/decrease b. right/decrease c. left/increase d. right/increase One liter of a solution is found to contain 0.022 moles of HCl. Calculate the pH of the solution. Assume complete dissociation. a. pH = 0.022 b. pH = 1.00 c. pH = 2.2 d. pH = 1.66 A Ka can be calculated for some chemical reactions. The Ka is a. the Keq for the reaction to the right. b. the Keq for the reaction to the left. c. the Keq for the dissociation of an acid. d. the pH of a very weak solution. Acidic solutions have an H+ concentration a. greater than the OH- concentration and a pH greater than 7.0. b. greater than the OH- concentration and a pH less than 7.0. c. less than the OH- concentration and a pH greater than 7.0. d. less than the OH- concentration and a pH less than 7.0. Give the IUPAC name of the following carboxylic acid? a. 4-propylnanoic acid b. 3-octyl-butanoic acid c. 4-butyl-3-methylheptanoic acid d. 3-methyl-4-propyloctanoic acid How many alkane constitutional isomers exist with the formula C5H12? a. 2 b. 3 c. 4 d. more than 5 Which compound is not an amine? Which of the following pairs of compounds represent constitutional isomers? a. 2-methylpropane and Pentane b. 2,2-dimethylbutane and 3-methylpentane c. 2,2-dimethylpropane and 2-methylpentane d. 2-methylbutane and 2-methylpropane Water soluble amines form solutions with a pH that is greater than 7. True False For a buffer to continue to work effectively, the pH of a buffer has to be close to the pKa of the conjugate acid True False The Ka for the reaction of acetic acid and water shown below is 1.8 x 10-5. Which of the following statements is true at pH 7? a. there is much more acetic acid than acetate ion b. there is more acetate ion than acetic acid c. the concentration of acetate ion is equal to that of acetic acid d. the pH is lower than pKa of acetic acid The effect of a catalyst on an equilibrium is a. to speed up the reaction to the left. b. to speed up the reaction to the right. c. to speed up the reaction equally in both directions. d. to shift the reaction in the direction of the catalyst. Calculate the pH of a solution containing 0.15 mole KOH dissolved in enough water to produce 2L solution a. pH = 0.075 b. pH = 0.15 c. pH = 1.12 d. pH = 12.88 One characteristic of basic solution is that a. the solution would turn litmus red. b. the solution would have a slippery feel. c. the solution would have a sour taste. d. the solution would dissolve some metals. This compound is mescaline, a hallucinogen. Which of the following statements is true? a. Mescaline is an amine. b. Mescaline contains an alcohol group. c. Mescaline is a carboxylic acid. d. Mescaline is an amine and contains an alcohol group. Carbon must form how many bonds? a. 1 b. 2 c. 3 d. 4 An amine will be converted into its conjugate acid by a. application of heat. b. reaction with an oxidizing agent. c. reaction with a strong acid. d. reaction with a strong base. The organic product formed when propanoic acid reacts with ethanol in the presence of an acid catalyst is: a. ethyl propanoic acid. b. ethylpropanoate. c. propylethanoate. d. propylethanoic acid. A 4º ammonium ion has four carbon atoms attached to a nitrogen atom causing it to have a 1+ charge. True False Which of the following is not a common characteristic of a base? a. it turns litmus pink/red b. it feels slippery c. it tastes bitter d. all of these choices are common characteristics of bases A buffer is capable of reducing the effect of the addition of small amounts of hydronium or hydroxide ion to a solution. True False In the Haber process for the production of ammonia, Which will not increase the amount of ammonia at equilibrium? a. increasing the concentration of N2 b. increasing the concentration of H2 c. increasing the concentration of N2 and H2 d. addition of a catalyst In the equation: a. H2CO3 and H3O+ are conjugate pairs b. H2CO3 is not amphoteric c. H2CO3 and HCO3 - are conjugate pairs d. H2CO3 and H2O are conjugate pairs Carboxylic acids, when compared with other molecules of similar size, a. have relatively lower melting points. b. have relatively higher boiling points. c. have a smaller number of hydrogen bonds. d. have more nonpolar locations. Which of the following lowers the pH when added to water? a. ethyl alcohol b. propanoic acid c. methyl amine d. methyl alcohol The chapter refers to physiological pH, which is approximately a a. pH of 6.3. b. pH of 7. c. pH of 8. d. pH of 10. Hydocarbons are organic compounds that a. contain carbon and hydrogen atoms only. b. are soluble in water. c. are polar molecules. d. contain carbon, hydrogen, and halogen atoms. Predict the organic product formed when heat is applied to the compound below: The reaction below is an example of what kind of reaction? a. neutralization b. hydrolysis c. esterification d. saponification [Show More]
Last updated: 2 years ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Feb 01, 2021
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Feb 01, 2021
Downloads
0
Views
122




.png)
.png)
.png)
.png)
.png)
.png)
.png)












